 |
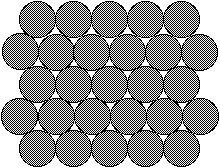
Solid - In a solid the particles
are packed closely together in a regular manner. Motion
is restricted by adjacent particles, and movement is
limited to vibrations. This arrangement explains the
difficulty solids have in being compressed and their
definite shape and volume.
|
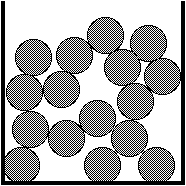 |
Liquid - At the melting point of
the solid the particles break loose from fixed positions
and can move relative to each other. The particles are
still packed closely together, but there is more space
for movement. The liquid takes up more space than the
solid (usually). The increased movement allows liquids to
flow. The ability of the particles to move relative to
each other accounts for variable shape liquids can take.
|
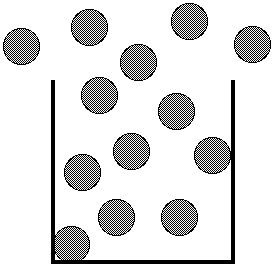 |
In the gas phase the particles are
well separated and move rapidly and randomly about.
Because the gas particles no longer attracted to each
other the gas expands to fill the container. The large
amount of space between gas particles explain the
compressibility of gases.
|