Lecture, Friday, August 30, 2002
We started this lecture with a review of todays PLE. Everyone seems to have
a good understanding of how to determine protons, electrons and neutrons using
isotopic notation for atoms (PLE5.1 and 5.2). No problems with identifying
metals and nonmetals in the periodic table.
Most everyone is able to read a formula, however we did have a little problem
with the number of oxygen molecules in C6H12O6.
Many students thought 3 when actually there are no O2 molecules
in the compound. Formula only tell us numbers of atoms of each element.
Finally the last problem in the PLE was a little challenging for some students
so we worked it out in class.
Problem #1:
Only two istopes of copper are known to exist, 63Cu has a isotopic
mass of 62.9295989 u and 65Cu has a isotopic mass of 64.9277929
u. The percent abundance for 63Cu is 69.17% and for 65Cu
the percent abundance is 30.83%. Calculate the atomic weigh, in atomic mass
units (u) for the element copper.
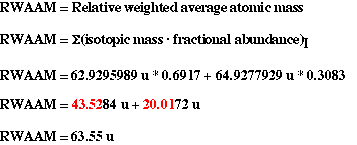
Next we talked about the periodic table defining rows, periods
and groups. We named the alkali metals, alkaline earth metals, transition
metals, boron, carbon, nitrogen, chalogen, halogen and inert gases.
We identified metals, nonmetals and metalloids.
Then we started talking about compounds, specifically ionic
and covalent compounds. I talked about the properties of two substances, NaCl
and glucose (C6H12O6).
Everyone should not be able to do problems PS2.1 - PS2.7. We'll
finish the last three problems in class on Wednesday.