With today's PLE we enter a new area of manipulating heat and how it is transferred
in chemical systems.
I asked you to
1. When 50. grams of water at 75 degrees Celsius is mixed with 50. grams of
water at 25 degrees Celisus, what is the final temperature, assuming no heat
is lost to the surrounding?
To solve this problem we have to think about the first law of thermodynamics
that states that energy is conserved. Any energy lost must be gained by something
else.
We have been using this idea, we'd just not discussed it specifically. For example
when we heat a sample of water in the solid phase at -10 ¾C to water in the
vapor phase at 110 ¾C the heat that is absorbed by the sample of water (the
system) is taken from the surroundings. The 1st law of thermodynamics says that
the energy used by the water (absorbed by the system) is exactly equal to the
heat given up by the surroundings.
In the problem in today's PLE we use the first law to solve this problem. In
this case there are two beakers of water each with the same mass, but different
initial temperatures. One sample is at 75 ¾C and the other is at 25 ¾C.
Our intuition knows that when the two samples are mixed the sample with the
higher temperature will decrease in temperature and the temperature of the water
at the lower temperature will rise. So one sample absorbs heat and the other
sample releases (looses) heat. But according to the first law the heat gained
by the sample at the lower temperature must equal the heat released by the sample
at the higher temperature.
One important assumption is made when doing this kind of problem….no heat
is lost to the surroundings when the two samples are mixed. This only occurs
under ideal conditions and normally some heat is lost to the surroundings.
Mathematically we express that as;
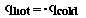
But we also know that for when dealing with heat for liquid water
that;
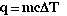
This equation relates the amount of heat gained or lost by a
sample of water in terms of the mass of the sample of water, its specific heat
and the change in temperature the sample undergoes.
So we can substitute that into our first equation (1st law of thermo);

So how do we handle using a different substance? We do the problem the exact
same way. On the PLE we used ethyl alcohol.
When 50. grams of water at 75 degrees Celsius is mixed with 50. grams of ethyl
alcohol at 25 degrees Celsius, what is the final temperature, assuming no heat
is lost to the surrounding?
