Lecture notes for Friday, August 24, 2001
Evaporation
Imagine a sample of a liquid in a beaker left out in a warm room for an extended
period of time. What happens to the liquid in the beaker? It evaporates, that
is, if we left the beaker there and returned after several days we would expect
all the liquid to be gone. Where did it go? Into the atmosphere. How does the
liquid, like water, at room temperature, evaporate?
How do we understand the nature of evaporation? Can we use the kinetic molecular
model to explain evaporation? And if we can, what new properties might the model
lead us to consider?
In a sample of a liquid the particles are in constant, chaotic motion (here
is the animation we used in class). At the particular
temperature the particles of a liquid have an average kinetic energy. But remember
there is a distribution of energies do that some particles have a high energy
and some have low energy. If a particle in the liquid phase has enough energy
and its velocity vector is oriented in the proper direction it can escape into
the vapor phase. Even though vaporization is an endothermic process since the
liquid is in a large room at constant temperature the room maintains the temperature
of the liquid. If the beaker is left open to the atmosphere eventually all of
the liquid evaporates. If we place a lid over the beaker to prevent the escape
of the water vapor in the room the volume of the water will not change. In this
case the water molecules escape into the air above the surface of the liquid.
After a while, when the number of molecules of water in the air above the liquid
become large, some molecules in the vapor phase condense by colliding with the
liquid. After a period of time the rate of escape of molecules from the liquid
phase equals the rate of condensation of vapor into the liquid phase. When this
occurs the system is in equilibrium. That is the two rates are equal, and there
is no net change. If a particle in the vapor phase condenses, an instant later
a particle evaporates. The system is in a state of dynamic equilibrium. That
is particles are constantly changing from vapor to liquid phase and visa versa.
However, if we measure the vapor pressure of the sample we see there is no change
in pressure over time. The pressure exerted by the water molecules in the vapor
phase, above the liquid, is called the vapor pressure of the liquid at the particular
temperature.
From the particulate level animation of a liquid we have three definitions
for vapor pressure. All are appropriate, but can be used depending on the question
asked. Vapor pressure is defined as;
1. the pressure exerted by the vapor above its liquid at a particular temperature;
2. the point at which the rate of condensation equals the rate of evaporation
(equilibrium vapor pressure);
3. the maximum pressure exerted by the vapor above its liquid at a given
temperature.
Next we continued our discussion of vapor pressure of liquids by turning
our attention to a demonstration setup showing three barometers. (Here is an
animation of the demonstration we performed live
in class.) The glass tubes contained mercury. No (ideally) air molecules are
found in the space above the mercury, i.e. a vacuum.
A sample of liquid introduced at the bottom of the column the liquid will
rise up the tube to the top of the mercury, because of density differences.
When the reaches the space above the mercury the liquid will immediately vaporize
to an extent equal to or less than the vapor pressure of the liquid at the particular
temperature. Whether the pressure exerted is equal to the vapor pressure of
the liquid depends on the amount of liquid injected. If we introduce water,
ethanol and ether into different columns we can see the difference in the vapor
pressure, at room temperature of each liquid. Water first. Notice the difference
in the height of the column of mercury after introducing the water sample. The
change in height is equal to the vapor pressure of the water (23.7 mmHg) at
23 degrees C. Notice there is a small amount of liquid water resting on the
surface of the mercury. Next we'll try ethanol. Notice the vapor pressure of
ethanol is greater at the same temperature (55 mmHg). Finally we tried ether.
First a small amount. We noticed the height of the column of mercury, but also
notice that there was no liquid on the surface of the mercury. This meant that
all of the liquid introduced vaporized. It all vaporized because the resulting
pressure exerted by the sample of ether is smaller than the vapor pressure of
ether. So more ether had to be added. And the level of the mercury drops even
further. Finally it has reached a level equal to the vapor pressure (diethyl
ether it is 520 mmHg).
Next we looked a plot of vapor pressure (y-axis) versus temperature (x-axis)
and observed the exponential increase in vapor pressure with temperature. We
also discussed the definition of a boiling point. The correct definition of
boiling point is the temperature at which the vapor pressure of a liquid equals
atmospheric pressure. With this definition we can understand why liquids boil
at lower temperatures when heated at high altitudes. At high altitudes the atmospheric
pressure is lower, there is less atmosphere above the surface at high altitudes.
If the atmospheric pressure is lower water will boil at a temperature at which
the vapor pressure is equal to the atmospheric pressure. This is a lower temperature.
I can get water boiling at room temperature. How hot is water boiling at room
temperature?
The normal boiling point of a liquid is the temperature at which the vapor
pressure equals 1 atmosphere.
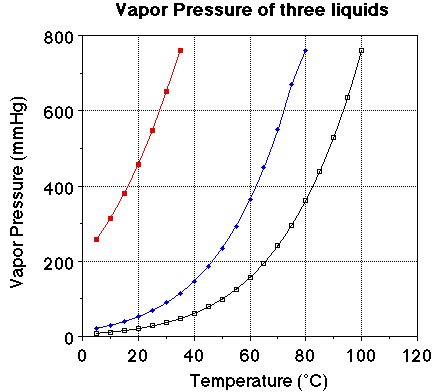
We we looked at the plot of vapor pressure versus temperature we noted the
plot of the three liquids were very similar. The obvious difference is the boiling
point for the three liquids. The vapor pressure of each of the liquids at 25
degrees C should be noted. The vapor pressure of water at 25 degrees C is 23.7
mmHg, for ethanol it is about 55 mmHg and for diethyl ether it is 520 mmHg.
Notice the boiling points for the three liquids. Water boils at 100 degrees
C, ethanol at 78. 3 degrees C and ether at 34.6degrees C. At a given temperature
a comparison of the vapor pressure suggests the liquid with the highest vapor
pressure will have the lower boiling point. At a given temperature a high vapor
pressure indicates there is a large number of molecules of the substance in
the vapor phase. The more molecules in the vapor phase at a given temperature
the easier it is for the molecules to escape into the vapor phase. The greater
the number of molecules in the vapor phase at a given temperature the weaker
the attractive forces between the molecules and the lower the energy required
to vaporize the molecules. That is there is a relationship between the heat
of vaporization and the vapor pressure of a liquid.