Lecture Notes, Wednesday, August 29, 2001
On several occasions up to this point I have used the term 'intermolecular
attractive forces'. I first used it when we looked at the animation of the container
of gas as it was cooled. (See the phase transition animation
at the particulate level.) Recall in this animation a container of gas particles
was cooled. As the temperature of a collection of particles was lower we observed
the particles slowing down. At the lower velocities colliding particles appeared
to stick together forming groups of particles. As the temperature continued
to drop the number of particles in these groups increased. Eventually the groups
of particles are of sufficient size that they fall to the bottom of the container
as a result of force of gravity, forming a liquid. As the tmperature continues
to drop the particles become more ordered, and their translational energy drops
to a very small value and a solid forms. Condensation occurs when the intermolecular
attraction between a pair of particles exceeds the kinetic energy of the collision.
The 'stickyness' exhibited by particles at the lower temperatures, which
result in the formation of liquids and eventually solids is due to intermolecular
attractive forces. Intermolecular means between molecules. Intramolecular means
between atoms. Intramolecular forces are what we call covalent bonds and are
very strong (100 - 1000 kJ/mol) (see also
Table 9.2 in Silberberg). Intermolecular forces are between molecules and are
weak (0.1 - 40 kJ/mol). Intermolecular forces are less directional compared
to covalent bonds and operate over a longer range compared to covalent bonds.
It is intermolecular forces which explain the formation of liquids and solids
in covalent compounds. Intermolecular attractive forces are electrostatic in
nature.
Intermolecular forces are classified into the following categories;
* ion-dipole (we will discuss when we cover Chapter 13)
* dipole-dipole
* induced dipole-induced dipole (London dispersion forces)
* hydrogen-bonding
To determine which type(s) of intermolecular attractive forces occur for
a particular substance the most important characteristic to determine for the
substance is its polarity. A polar molecule possess a permanent dipole moment
(see pages 384 - 386 in Silberberg) as a result of its molecular shape and from
the unequal sharing of electrons in chemical bonds which produces the separation
of charge which produces the dipole.
To recognize a polar substance (you have to do this on the review problem
set) you must draw the Lewis structure (see pages 361 - 371 in Silberberg, CHEM
1314 lecture notes). After drawing a Lewis structure look at the central
atom.
Rules for predicting whether a molecule is polar (has a permanent dipole)
or is nonpolar
-
If the central atom has one or more unshared, nonbonding, pairs of electrons
the molecule is most likely polar. Examples include; NH3, H2O,
SO2
-
If the central atom has no unshared, or nonbonding, pairs of electrons
and nonidentical terminal atoms the molecule is polar.
-
If the central atom has has no unshared, or nonbonding, pairs of electrons
and the terminal atoms are identical the molecule is nonpolar.
A simple example is HCl. The Lewis structure for HCl is;
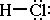
The pair of electrons in the covalent bond between hydrogen and chlorine is
unequally shared due to the difference in electronegativity between hydrogen
and chlorine. Chlorine has a greater electronegativity compared to hydrogen
and as a result the electrons in the covalent bond spend a greater proportion
of the time closer to the chlorine nucleus. So for chlorine the electrons in
the covalent bond spend more time nearer its nucleus producing a small partial
negative charge in the molecule. Since the hydrogen atom 'sees' the electron
in the covalent bond less frequently it has a partial positive charge. This
permanent separation of some small amount of charge on the HCl molecule produces
a permanent dipole. Here is a picture of the charge on a space-filling model
of the HCl molecule;
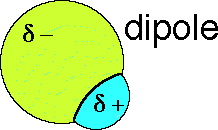
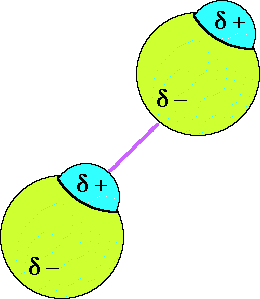
A collections of HCl molecules will align themselves such that the negative
end of one HCl molecule is attracted to the positive end of an adjacent HCl
molecule.
The ion-dipole force results from the attraction of an ion of positive or
negative charge and the oppositely charged end of the dipole on the polar molecule.
The strength of the attraction depends on the magnitude of the charge on the
ion, the magnitude of the dipole moment and the distance between center of the
ion and the midpoint of the polar molecule. Ion-dipole forces are important
for ionic compounds which dissolve in water.
Dipole-dipole forces of attraction occur between polar molecules. This type
of attractive intermolecular force occurs between the polar molecules of a pure
substance, such as HCl, or between two different polar molecules. Here is an
animation depicting the attraction when two HCl
molecule approach each other. The attraction arises because of the permanent
dipole in the polar molecule. Dipole-dipole attractions are relatively weak
compared to ion-ion attractions, because the charges on polar molecules are
generally quite small. In liquids the molecules are free to move relative to
each other, but will do so such that sometimes orientations of adjacent molecules
are attractive and sometimes they are repulsive. The overall average effect
is attractive.