Lecture Notes for Friday, September 21, 2001
Colligative Properties
It turns out that for dilute solutions of nonvolatile solutes
the  Tfp and the
Tfp and the  Tbp
are proportional to the molality of the solution.
Tbp
are proportional to the molality of the solution.
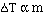
to get an equality we add a constant and
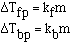
The magnitude of the constants are different for a particular
solvent and also vary with different solvents. The units on 'k', the freezing
point constant or the boiling point constant, are degrees C m-1.
For water the freezing point constant is 1.86 degrees C m-1 and the
boiling point constant is 0.512 degrees C m-1. A 1 molal aqueous
solution of any nonvolatile nonelectrolyte boils at 100.512 degrees C and melts
at Ð1.86 degrees C. So if we have a concentration of a nonvolatile solution
other than 1 molal we can use either equation to calculate the new boiling or
freezing point. It must be remembered that when solving for ÆT that if the freezing
point is expected the ÆT must be subtracted from 0 degrees C (freezing point
depression) and added to 100 degrees C (boiling point elevation).
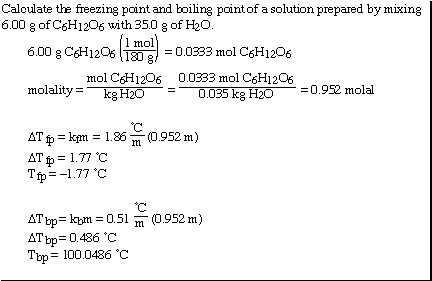
Another sample problem;

We can also use the expression to determine the molecular weight
of an unknown solute. If we recognize that;
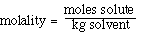
and that;

substituting into the freezing point expression we have;
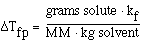
rearranging and solving for MW we have;
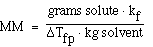
Another more interesting example;
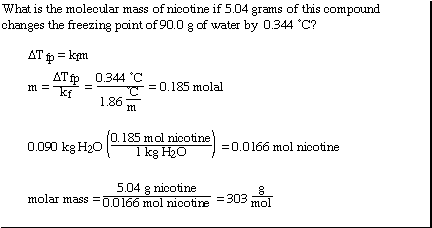
We can also use the colligative properties to help understand
the behavior of strong electrolytes as well as nonelectrolytes. If we look at
some experimental freezing point data some interesting information can be obtained.
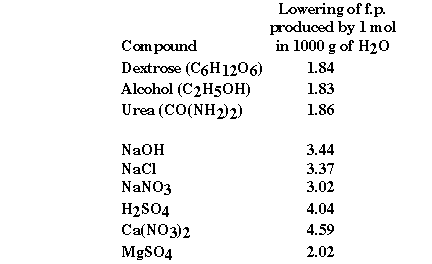
What is it about the second group of compounds that would explain
the observed freezing point depressions? They are all ionic compounds! They
are what we have called strong electrolytes, because we understand these compounds
to completely dissociate in aqueous solution. But do they?
Experimentally the  Tfp
for NaOH is 3.44 degrees C. Ideally we would expect that all of the NaOH would
dissolve according to the reaction:
Tfp
for NaOH is 3.44 degrees C. Ideally we would expect that all of the NaOH would
dissolve according to the reaction:
NaOH(s) ÐH2O--> Na+(aq) + OH-(aq)
When an ionic compound dissolves in water the ionic compound
exists as ions in solution. 1 mol of NaOH dissolves to form 2 moles of ions
(particles). Inorder to deal with this behavior we need a variation on the freezing
point expression. It is;
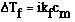
where;

Ideally we would predict that NaOH would completely dissociate
so that 'i' would have a value of 2 and the theoretical freezing point would
be 3.72 degrees C. We experimentally observe that all the NaOH dissolves yet
the freezing point depression data suggest that not all of the NaOH has dissociated.
Some of the NaOH remains associated. Based on the experimental change in freezing
point the experimental value of 'i' is 1.85. In general, the more concentrated
the solution the greater the observed deviation from ideality. As the solution
becomes more dilute the experimental 'i' value approaches the theoretical value.
The difference between the ideal value of 'i' and the observed is due to something
we call 'ion-pairing'. In an aqueous solution the ions of the solute are surrounded
by water molecules. If the the solution is very dilute the large number of water
molecules that surround the ions effectively prevent the ions from seeing each
other. All an individual ion sees is other water molecules. At higher concentrations
of ions the number of water molecules available for solvation is lowered and
there are fewer water molecules surrounding any particular ion. The result is
the ions can 'see' each other and they feel some attraction and they will 'pair
up' in solution. When they pair there are fewer particles in the solution and
'i' drops from it ideal value.
We can use experimental 'i' values obtained by measuring the
freezing point depression of electrolytes to determine whether the substance
is strong, weak or a nonelectrolyte. For example, when the freezing point of
a 0.100 molal solution of HCl is measured the observed value is 0.353 degrees
C. Is HCl an electrolyte? If it were not the expected change in temperature
would be 0.186 degrees C. Based on our experimental data HCl is in fact a strong
electrolyte and is 90 % dissociated. On the other hand a 0.100 molal solution
of HC2H3O2 has a  Tf
of 0.195 degrees C, very close to 0.186 degrees C. Acetic acid is considered
to be a weak electrolyte, while dextrose and urea are considered to be nonelectrolytes.
With more understanding of this kind of problem we would be able to calculate
the percent dissociation of the compound.
Tf
of 0.195 degrees C, very close to 0.186 degrees C. Acetic acid is considered
to be a weak electrolyte, while dextrose and urea are considered to be nonelectrolytes.
With more understanding of this kind of problem we would be able to calculate
the percent dissociation of the compound.
Sample Problem:
Calculate the freezing point and the boiling point of a saturated
solution of Li2CO3. The solubility of lithium carbonate
is 0.72 g per 100 g of water at 100 degrees C.
Try another sample problem:
2.57 g of an ionic compound with the formula KX are dissolved
in 120 g of water. The freezing point of the solution was lowered by 1.37 degrees
C. Determine the formula weight of X.