Lecture Notes for Monday, September 24, 2001
Did you ever wonder why some chemical reactions proceed lighting fast, while
others take months, years or even thousands of years? Mixing potassium metal
with water results in fast, violent reaction. Yet the decomposition of carbon
in the form of diamond to carbon in the form of grahite takes thousands of years.
Did you ever wonder how catalysts work to increase the speed of a chemical reaction?
Or why changing the temperature has such a dramatic change in the speed of a
reaction?
Chemical kinetics is the study of the rates and mechanisms of chemical reactions.
Measuring the rate or speed of a chemical reaction is straight forward. Remember
speed is a change in distance with time. Speed in chemical reactions is simply
the change in the amount of a reactant or product with time. The way this information
is obtained in the laboratory will depend on the particular reaction that is
performed but generally consists of using some technique to measure the concentration
of one of the species in a chemical reaction over time. For example, if in a
particular reaction one of the reacting chemicals undergoes a color change it
would be possible to measure the intensity of the color every minute. If a gas
was produced in a chemical reaction it would be possible to measure the volume
of gas produced over time, with the correct equipment. A little later in todays
lecture I'll introduce how we measure the speed, or rate of a chemical reaction
with this type of information. But what do we do with this experimental information
once we have collected it? Up to this point in your chemistry careers we have
been learning about chemical reactivity and chemical principles. We are, for
instances, familiar with a many of things that a chemical equation tells. Look
at the Pre-Lecture Exploration
for Monday, September 24, 2001 before continuing.
For example the equation;
NO(g) + O3(g) ---> NO2(g) + O2(g)
What do we know about this reaction?
Balance
Stoichiometry
Heat of Reaction
For example the equation;
H2(g) + Br2(g) ---> 2HBr
How does this reaction occur?
For example the equation;
HBr(g) + O2(g) ---> H2O(g) + Br2(g)
Although we have never seen this particular equation before we still recognize
many features about the equation. First, the reaction is not balanced;
4HBr(g) + O2(g) ---> 2H2O(g) + 2Br2(g)
It is a combustion reaction so we would predict that the reaction occurs with
a liberation of heat. We are capable of calculating 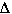 Hrxn
for the reaction if we were give the ÆHf for each of the reactants
and products. For the reaction
Hrxn
for the reaction if we were give the ÆHf for each of the reactants
and products. For the reaction 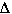 Hrxn
is -338 kJ/mol. We can apply our understanding of stoichiometry (mole relationships)
to determine the amounts of reactants and products involved in a chemical reaction.
All of these things that I have mentioned are derived from the chemical equation.
We are only concerned about what the reactants and what the products are, we
do not need to know what happens to the individual molecules during the course
of the reaction.
Hrxn
is -338 kJ/mol. We can apply our understanding of stoichiometry (mole relationships)
to determine the amounts of reactants and products involved in a chemical reaction.
All of these things that I have mentioned are derived from the chemical equation.
We are only concerned about what the reactants and what the products are, we
do not need to know what happens to the individual molecules during the course
of the reaction.
Chemical kinetics concerns itself with what happens while a reaction is underway,
not just in the final outcome. Chemical kinetics does not change the chemical
reaction it simply provides a clearer picture of what happens during the progress
of the chemical reaction. With the new knowledge it will be possible to control
the conditions of a reaction which will help increase the amount of the desired
products. If we were able to travel to the molecular level and watch the progress
of the reactants in the course of the reaction and the many changes they undergo
in proceeding to the products we would be doing chemical kinetics. If we were
able to observe first hand how the electrons behaved during a chemical reaction
we would be doing chemical kinetics.
But, unfortunately, we can not 'see' molecules or electrons with our own eyes
so it is impossible to view these molecular and electronic interactions firsthand.
What we do instead is to use instruments that follow the reaction, by watching
properties of particular reactants that depend upon their concentration. This
way we can watch how a reactant disappears in a chemical reaction. Or we can
watch the properties of a particular product that depend upon concentration
and we can see how its concentration changes during the course of a chemical
reaction. Then by applying mathematical models and doing some calculations it
is possible to understand the pathway a reaction follows. The best way to try
to understand reactions in this way is to study how fast the reactions occur-to
study the rate of a chemical reaction.