We started lecture viewing the reaction between sodium
metal and chlorine gas. The direct reaction between sodium and chlorine
can be slow until a fresh surface of the sodium metal is exposed. Adding a very
small amount of water is generally sufficient to react with the sodium metal
to expose fresh surface for reaction with chlorine. The produce is solid sodium
chloride.
Next we viewed an animation showing a particulate
level view of the three phases as the temperature of a sample is cooled.
A container of gas particles was cooled. As the temperature of a collection
of particles was lower we observed the particles slowing down. At the lower
velocities colliding particles appeared to stick together forming groups of
particles. As the temperature continued to drop the number of particles in these
groups increased. Eventually the groups of particles are of sufficient size
that they fall to the bottom of the container as a result of force of gravity,
forming a liquid. Condensation occurs when the intermolecular attraction between
a pair of particles exceeds the kinetic energy of the collision. As the temperature
continues to drop the particles become more ordered, and their translational
energy drops to a very small value and a solid forms.
The 'stickyness' exhibited by particles at the lower temperatures, which
result in the formation of liquids and eventually solids is due to intermolecular
attractive forces. Intermolecular means between molecules. Intramolecular means
between atoms. Intramolecular forces are what we call covalent bonds and are
very strong (100 - 1000 kJ/mol). Intermolecular forces are between molecules
and are weak (0.1 - 40 kJ/mol). Intermolecular forces are less directional compared
to covalent bonds and operate over a longer range compared to covalent bonds.
It is intermolecular forces which explain the formation of liquids and solids
in covalent compounds. Intermolecular attractive forces are electrostatic in
nature.
Intermolecular forces are classified into the following categories;
* ion-dipole
* dipole-dipole
* induced dipole-induced dipole (London dispersion forces)
* hydrogen-bonding
We will discuss these different intermolecular attractive forces in a few
lectures.
In Pre-lecture exploration #2
we watch what happened to the temperature of a sample of water as heat was added
at a constant rate. The heating curve is summarized in the graph below;
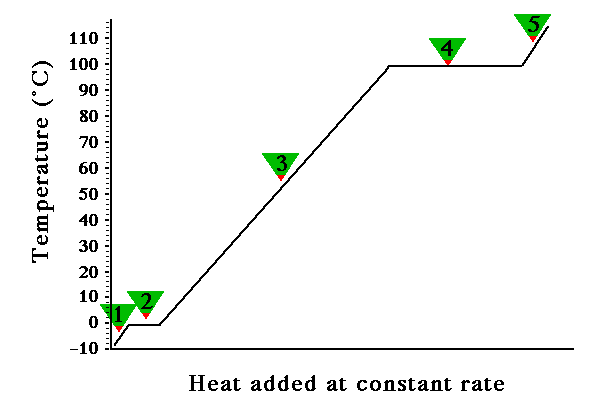
We notice the obvious, as heat is added (along the x-axis) the temperature
of the sample, in general, increases. Looking at the plot more closely we actually
see there are two plateaus where the temperature remains constant as heat is
added. The sample is water so it is interesting that the two plateaus are at
0 degrees Celsius and 100 degrees Celsius. One plateau (#2) corresponds to the
melting/freezing point of water, the other plateau (#4) corresponds to the boiling/condensing
point of water. Besides the two plateaus there are three regions where the temperature
increases linearly with added heat.
The five label regions can be characterized by specifying the temperature
change (if any) and the phase(s) present.
At #1 the temperature changes from -10 degrees Celsius to 0 degrees Celsius;
the phase is H2O(s). We are referring to the line with a positive
slope between - 10 deg Celsius and 0 deg Celsius. So as heat is added the temperature
of the sample of water increases linearly. That the line has a constant slope
over the temperature range suggests uniform behavior. That is there is a relationship
between the amount of heat added and the temperature change. It turns out that
how much heat is required to change the temperature of solid water also depends
on the amount of water. The characteristic property that relates the amount
of heat required to change the temperature of 1 gram of a substance by 1 degree
Celsius is called specific heat. The specific heat of water in the solid phase
has a value of 2.09 J g-1 C-1.
At #2 the temperature does not change, but heat is added. The added heat is
absorbed by the solid converting the solid to the liquid. So at the left most
point on the horizontal line the sample is all solid and the temperature is
0 degrees Celsius. As heat is added the solid melts, recall it takes energy
to over come the attractive forces holding the particles together in the solid
phase, forming the liquid. The amount of intermolecular attractive forces broken
in this phase change is small since the particles are very close to each other
in each phase. So at the right most point of the horizontal line the sample
is all liquid at 0 degrees Celsius. The heat required to convert water from
the solid phase to the liquid phase;
H2O(s) ---> H2O(l)
is called the heat (enthalpy) of fusion (heat of solidification). For water
the heat of fusion has a value of 6.01 kJ mol-1.
At #3 the temperature changes from 0 degrees Celsius to 100 degrees Celsius.
The phase is liquid. Again notice the linear behavior. In this temperature range,
given the amount of water remains constant, the addition of a given amount of
heat causes the temperature of the water to change by the same amount. The specific
heat of liquid water is 4.184 J g-1 C-1. Comparing liquid
water to solid water it takes more than twice the amount of heat to change the
temperature of liquid water by the same amount compared to solid water.
At #4 the temperatrue does not change as the heat is added. At this plateau
the heat added is absorbed by the liquid as it is converted to the gas(vapor)
phase. The heat absorbed is used to overcome the attractive forces between the
particles in the liquid phase. Notice the amount of energy required to convert
a liquid to its gas is much greater compared to the energy required to convert
a solid to its liquid. At the left-most point on this line the sample is all
liquid at 100 degrees Celsius. Moving to the right the amount of water in the
liquid and vapor change, and the temperature remains constant. At the extreme
right the sample is all vapor at 100 degrees Celsius.The heat required to convert
water from the liquid phase to the gas phase;
H2O(l) ---> H2O(g)
is called the heat (enthalpy) of vaporization (heat of condensation). For
water the heat of vaporization has a value of 40.67 kJ mol-1.
At #5 the temperature changes from 100 degrees Celsius to 110 degrees celsius.
The phase is gas(vapor). In the vapor phase the specific heat of water is 1.84
J g-1 C-1.
Here is a summary table of the specific heats and enthalpy of phase changes
for water;
Specific Heat of H2O(s)
|
2.09 J g-1 C-1
|
Specific Heat of H2O(l)
|
4.184 J g-1 C-1
|
Specific Heat of H2O(g)
|
1.84 J g-1 C-1
|
Enthalpy of fusion
|
6.01 kJ mol-1
|
Enthalpy of vaporization
|
40.67 kJ mol-1
|
Let's look at several sample problems;
How much heat is required to change 36.0 g of H2O(l) at 100 deg
C to 36.0 g of H2O(g) at 100 deg C? Answer
How much heat is required to convert 30.0 g of H2O(s) at -10 deg
C to 30.0 g of H2O(g) at 110 degC. Answer
The specific heat of iron is 0.433 J g-1 C-1. What is
the final temperature if 45.0 g of iron, initially at 95 deg C, is added to
100.0 g of water at 23.0 deg C? Answer.