The Lewis structure for water;
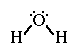
Water is polar. It has two lone-pairs of electrons on the central atom.
Can you locate the regions of partial negative and partial positive charge
on the water molecule?
Answer.

Because the oxygen atom is more electronnegative compared to the hydrogen
atom it has a greater attraction to the electrons in the O-H bond. Additionally
the lone-pairs of electrons on oxygen contribute to locating the partial negative
charge on the oxygen atom in the moelcule. Therefore the hydrogen atoms carry
a partical positive charge.
So what happens when several water molecules are located in close proximity?
Can you draw a picture using the Lewis structures depicting the orientation
of three water molecules to each other based on the partial charge carried on
the oxygen and the hydrogen atoms? Answer.