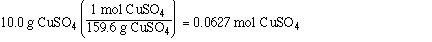Concentration Expressions/Definitions
Many reactions, and experiments are performed in solution. When doing quantitative
experiments we must know the exact amount of reagents involved in the reaction.
We can express the relative amount of a solute in a solution by expressing the
concentration of the reagent as a ratio of an amount of solute to the amount
of solvent. In your earlier experience in chemistry the concentration expression
you are familiar with is molarity.
Molarity is defined as

molarity is a ratio of the mol of solute to the volume of solution.
There are three additional concentration definitions that we
will discuss: weight %, mol fraction and molality. These new terms are defined
as;
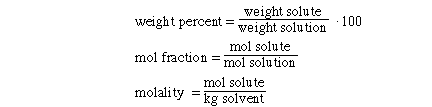
To see how we use these definitions we prepared a solution in
class by mixing 10.0 g CuSO4 with 90.0 g of H2O.
I. Weight Percent CuSO4
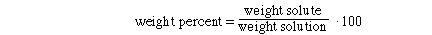
Substituting the amounts into the weight % expression and solving,
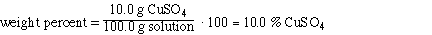
So the solution is 10.0% CuSO4. The weight % water
is 100 - 10.0 = 90.0%
II. Mol fraction CuSO4

First we need to determine the number of mol of solute and
solvent;
Substituting the amounts into the mol fraction and solving,
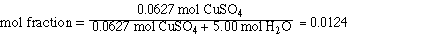
The mol fraction of H2O is obtained by subtracting
the mol fraction of CuSO4 from 1.00.
III. Molality of CuSO4

We know the mol of CuSO4 from the mol fraction calculation
and the mass of H2O.
0.0627 mol CuSO4
90.0 g of H2O
Substituting,
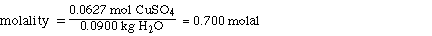
IV. Molarity of CuSO4

Determining the molarity of the solution requires additional
information. Initially we were given the mass of CuSO4 and the
mass of H2O. Adding the masses together to gives 100 g of solution
however, we can not say that the volume is 100 mLs of solution. To obtain
the volume of the solution we must know the density of the solution. Since
the solution is 10% CuSO4 we cannot assume the density of the solution
is the same as the density of water. When we mixed the 10.0 g of CuSO4
with 90.0 g of water the final volume of the solution was about 90.2 mLs.
So the mol of CuSO4 and the volume of solution are;
0.0627 mol CuSO4
90.2 mL of solution
Substituting,
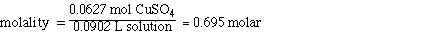
The four concentration expressions we will use are weight%, mol
fraction, molality and molarity. You must know these definitions. I will not
include them on the Useful Information page of the next exam. You must feel
comfortable using each of these concentrations and converting between them.
Let's consider a few problems to practice using these expression
and converting between them.
Problem I:
Calculate the molality and mol fraction of HCl for a solution
which has a mass percent of 37.1 %.
Show Me! (Help me by showing me some
steps to solve this problem.)
Answer (This is the answer)
Problem II:
If the density of the HCl solution is 1.18 g/mL, calculate the
molarity of the solution.
Problem III:
The density of a solution of Pb(NO3)2 prepared
by adding enough water to 24.0 g of solid Pb(NO3)2 and
diluting to a final volume of 100 mLs is 1.20 g mL-1. Calculate the
weight%, mol fraction, molality and molarity of Pb(NO3)2
in the solution.
Problem IV:
A solution of HF is 40.9 m. The density of the solution is 1.14
g cm-3. Calculate the molarity of the solution.