Calculate the freezing point and the boiling point of a saturated
solution of Li2CO3. The solubility of lithium carbonate
is 0.72 g per 100 g of water at 100 degrees C.
Answer:
We need to begin by calculating the molality of the saturated
Li2CO3 solution.
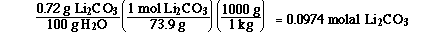
We know the molality of the Li2CO3 solution,
but we need to consider that Li2CO3 is a strong electrolyte.
Determine the number of particles Li2CO3 forms when dissolved
in water.
Li2CO3(s) -H2O--> 2Li+(aq)
+ CO32-(aq)
Every mol Li2CO3 that dissolves produces
3 mol of particles.
Calculate the change in freezing point and check your answer
here.