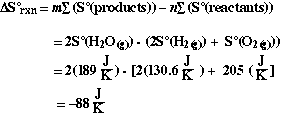Calculate 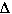 So
for a each of the following reactions,
So
for a each of the following reactions,
a) 2H2(g) + O2(g) ----> 2H2O(g)
Answer
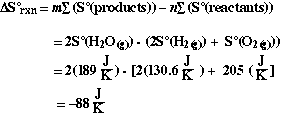
We notice that the calculated value is negative. Just as we predicted
on the basis of comparing the relative order/disorder of the reactants and products.
In this case we concluded 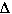 S was likely
to be negative becaue there are more moles of gas on the reactants side compared
to the product side.
S was likely
to be negative becaue there are more moles of gas on the reactants side compared
to the product side.