Predict whether 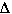 S
is positive, negative, or close to zero for the following changes.
S
is positive, negative, or close to zero for the following changes.
a) 2H2(g) + O2(g) ----> 2H2O(g)
Answer
In this example there are three moles of gases on the reactants
side and only two mole of gas on the product side. Even though the molecular
complexity of H2O(g) is greater than either H2(g) or O2(g),
the numbers of moles of gases will define the 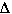 S.
S.
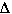 S for this reaction is negative,
the reactants are more disordered than the products.
S for this reaction is negative,
the reactants are more disordered than the products.