Using the table of entropy values above, calculate  So
for a each of the following reactions,
So
for a each of the following reactions,
b) 2C2H6(g) + 7O2(g) ---->
6H2O(g) + 4CO2(g)
Answer
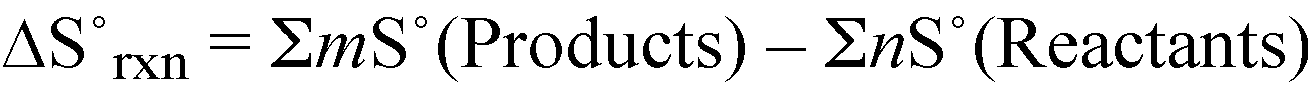
 S°rxn = 4S°(CO2(g)) + 6S°(H2O(l)) - [2S°(C2H6(g)) + 7S°(O2(g))]
S°rxn = 4S°(CO2(g)) + 6S°(H2O(l)) - [2S°(C2H6(g)) + 7S°(O2(g))]
 S°rxn = 4(213.6 J mol-1 K-1) + 6(69.9 J mol-1 K-1) - [2(186 J mol-1 K-1) + 7(205 J mol-1 K-1)]
S°rxn = 4(213.6 J mol-1 K-1) + 6(69.9 J mol-1 K-1) - [2(186 J mol-1 K-1) + 7(205 J mol-1 K-1)]
 S°rxn = (854.4 J mol-1 K-1) + (419.4 J mol-1 K-1) - [(372.7 J mol-1 K-1) + (1435 J mol-1 K-1)]
S°rxn = (854.4 J mol-1 K-1) + (419.4 J mol-1 K-1) - [(372.7 J mol-1 K-1) + (1435 J mol-1 K-1)]
 S°rxn = -533.9 J mol-1 K-1
S°rxn = -533.9 J mol-1 K-1
We notice that the calculated value is very negative. Just as
we should predict on the basis of comparing the relative order/disorder of the
reactants and products. There are 4 moles of product gases and 9 moles of reactant
gases. Also there are 6 moles of products in the liquid phase and no reactants
in the liquid phase. The products are much more ordered compared to the reactants.
We must conclude the  S of the reaction
is negative.
S of the reaction
is negative.