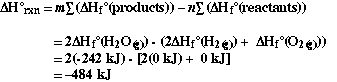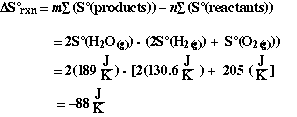Is the reaction below spontaneous at 298 K?
a) 2H2(g) + O2(g) ----> 2H2O(g)
Answer
First we can calculate  H
for the reaction,
H
for the reaction,
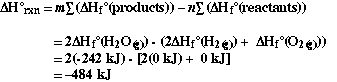
The  S was calculated
earlier and is shown below
S was calculated
earlier and is shown below
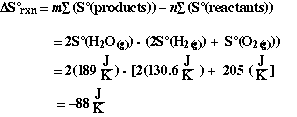
So now we have  H and
H and
 S.
S.  G
can now be calculated using the equation,
G
can now be calculated using the equation,
 Gsystem
=
Gsystem
=  Hsystem - T
Hsystem - T Ssystem
Ssystem
 Gsystem
= -484 kJ - 298 K(
Gsystem
= -484 kJ - 298 K(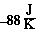 )
)
 Gsystem
= -484 kJ + 26.2 kJ = -458 kJ
Gsystem
= -484 kJ + 26.2 kJ = -458 kJ
So we conclude the following, the reaction is very exothermic
(-484 kJ), becomes more ordered (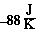 ),
but is still spontaneous. How could that be you might ask? Did you not say for
a reaction to be spontaneous the entropy had to increase?
),
but is still spontaneous. How could that be you might ask? Did you not say for
a reaction to be spontaneous the entropy had to increase?
And yes I did say that, but listen carefully, for a reaction
to be spontaneous the entropy of the UNIVERSE must increase! In this problem
the entropy of SYSTEM decreased. The reaction is spontaneous because the reaction
is VERY exothermic, releasing heat to the surroundings causing the entropy of
surrounding to increase much more than the entropy of the system decreased.
We notice that the calculated value is negative. Just as we predicted
on the basis of comparing the relative order/disorder of the reactants and products.
In this case we concluded  S was likely
to be negative because there are more moles of gas on the reactants side compared
to the product side.
S was likely
to be negative because there are more moles of gas on the reactants side compared
to the product side.