Lecture Notes for Wednesday, September 19, 2001
Chapter 13
Colligative Properties
Having discussed concentration units we need to extend the idea of solutions
to investigate how some physical properties of the solvent are effected upon
addition of a nonvolatile solute. A nonvolatile solute is a solute which has
little tendency to escape from the solution. A solution of a nonvolatile solute
(solid) has properties that are modified from the properties of the liquid (solvent).
The difference between the properties of the solution and those of the pure
liquid are a consequence of the number of solute particles present in the solution.
The properties which depend on the number of particles of solute are called
colligative properties.
These include;
1) Vapor Pressure lowering
2) Boiling Point elevation
3) Freezing Point depression
4) Osmotic Pressure
Colligative properties are properties which depend on the number of molecules
or ions of solute present, and not on what the particles are (as long as they
are not volatile). We will find colligative properties are important as they
will provide us with information about the number of particles of solute present,
and hence about molecular weights and degree's of dissociation (ionization)
in solution. Early chemists, such as Arrhenius, were able to show that for some
solutes more particles were present in solution than there were 'molecules'
of solute, and hence that the solute particles were breaking (dissociating)
into ions.
To understand the effect of a solute on the physical properties of a liquid, like vapor pressure, boiling point, freezing point and osmotic pressure we will consider the phase change of water and the  S of the phase change;
S of the phase change;
H2O(l) --> H2O(g) phase change for pure water
H2O(solution,l) --> H2O(g) phase change for a solution containing a nonvolatile solute
In the Figure below shown the entropy changes for the above phase change from liquid to gas for pure water and for the phase change from solution to gas when a nonvolatile solute has been added to water.
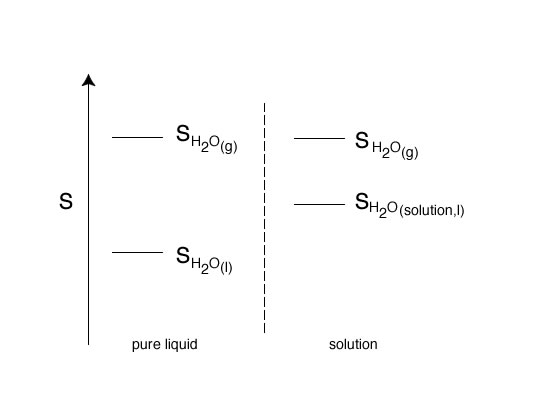
At a given temperature liquid water has a pressure exerted by water in the vapor phase above its liquid. The  S for the phase change helps determine the vapor pressure. Now consider adding a nonvolatile solute to the water (forming a solution) and consider the
S for the phase change helps determine the vapor pressure. Now consider adding a nonvolatile solute to the water (forming a solution) and consider the  S for the phase change. Since adding the solute to water the entropy of the solution is higher compared to the entropy of pure water. The entropy of water in the vapor phase is the same, so the result of adding a nonvolatile solute to water is to produce a smaller
S for the phase change. Since adding the solute to water the entropy of the solution is higher compared to the entropy of pure water. The entropy of water in the vapor phase is the same, so the result of adding a nonvolatile solute to water is to produce a smaller  S for the phase change. Since
S for the phase change. Since  S is smaller there is a lower tendency for water to move to the gas phase and the vapor pressure above the liquid is lower.
S is smaller there is a lower tendency for water to move to the gas phase and the vapor pressure above the liquid is lower.
Below is a table with the vapor pressure
of water upon addition of a solute. A pure
liquid, at a given temperature will be in equilibrium with its vapor. If we
consider water at 25 °C, the vapor pressure is 23.6 Torr. If we prepare solutions,
by adding a nonvolatile solute, containing different mole fractions of solvent
as shown below, and measure the vapor pressure due to water above the solution,
we would obtain the following data.
Mole Fraction (solvent)
|
Vapor Pressure (mm Hg)
|
1.0
|
23.600
|
.9
|
21.240
|
.8
|
18.880
|
.7
|
16.520
|
.6
|
14.160
|
.5
|
11.800
|
.4
|
9.440
|
.3
|
7.080
|
.2
|
4.720
|
.1
|
2.360
|
0
|
0
|
Graphing this data the plot looks like;

This is called Raoult's Law and is given as;

where;

Here is a sample problem using Raoult's law;

The equation;

can be rewritten in a slighlty different form to suggest another
useful technique. The mole fraction of solvent is given as;

Which can be further transformed to;

From this relationship it is possible to determine the molar
mass of a compound from the change in vapor pressure of the solvent.

The total vapor pressure above a solution is the sum of the
vapor pressures of the solution components (solute and solvent).

Substituting for Raoult's law we have;

The composition of the vapor is not the same as the composition
of the solution. Remember vapor composition, while it does depend on the mole
fraction of the component of the solution also depends on the vapor pressure
of the components. The vapor above a solution is always richer in the component
that has the higher vapor pressure at the given temperature.
<-----Notes are going to be changed---->
The explanation, based on entropy, of how a nonvolatile solute effects the vapor pressure of the
pure liquid can now be transferred to help us understand how the boiling point and melting point of
the solvent are changed. Below are two figures showing the entropy for boling point of water and for the freezing point of water.


Consider our old friend
 G =
G =  H - T
H - T  S
S
At the boiling point or at the freezing point  G = 0 so we can rearrange the equation to solve for the boiling point or for the freezing point;
G = 0 so we can rearrange the equation to solve for the boiling point or for the freezing point;
 G = 0 =
G = 0 =  Hvap - Tbp
Hvap - Tbp  Svap
Svap
Tbp =  Hvap /
Hvap /  Svap
Svap
Similarly,
 G = 0 =
G = 0 =  Hfus - Tfp
Hfus - Tfp  Sfus
Sfus
Tfp =  Hfus /
Hfus /  Sfus
Sfus
Looking at the figures depicting the entropy changes for the pure solvent and for a solution of a nonvolatile solute in the same solvent for the boiling point and the freezing point we see different behaviors.
For the boiling point case we see that the  Ssolution is smaller compared to
Ssolution is smaller compared to  S(H2O) so this would result in the Tbp of the solution being larger than the Tbp for the pure solvent. We see the normal boiling point is elevated as a result of the solute.
S(H2O) so this would result in the Tbp of the solution being larger than the Tbp for the pure solvent. We see the normal boiling point is elevated as a result of the solute.
However in the case of the freezing point notice the  Ssolution is larger compared to
Ssolution is larger compared to  S(H2O)so this would result in lower freezing point for the solution compared to the pure solvent. So the resulting solution has a new freezing point which lower than
the original freezing point.
S(H2O)so this would result in lower freezing point for the solution compared to the pure solvent. So the resulting solution has a new freezing point which lower than
the original freezing point.
It turns out that for dilute solutions of nonvolatile solutes
the  Tfp and the
Tfp and the  Tbp
are proportional to the molality of the solution.
Tbp
are proportional to the molality of the solution.

to get an equality we add a constant and

The magnitude of the constants are different for a particular
solvent and also vary with different solvents. The units on 'k', the freezing
point constant or the boiling point constant, are degrees C m-1.
For water the freezing point constant is 1.86 degrees C m-1 and the
boiling point constant is 0.512 degrees C m-1. A 1 molal aqueous
solution of any nonvolatile nonelectrolyte boils at 100.512 degrees C and melts
at Ð1.86 degrees C. So if we have a concentration of a nonvolatile solution
other than 1 molal we can use either equation to calculate the new boiling or
freezing point. It must be remembered that when solving for ÆT that if the freezing
point is expected the ÆT must be subtracted from 0 degrees C (freezing point
depression) and added to 100 degrees C (boiling point elevation).


