Lattice Energy: Strengths of Ionic Bonds
Although there are several important factors which must be considered in determining
the energy associated with forming ionic bonds, the lattice energy is where
we will focus. The lattice energy is defined as the energy required in the following
reaction;
MmXn(s) ---> Mn+(g)
+ Xm-(g)
Lattice Energies of Alkali Metal Halides (kJ mol-1)
| |
F-
|
Cl-
|
Br-
|
I-
|
Li+
|
1036
|
853
|
807
|
757
|
Na+
|
923
|
787
|
747
|
704
|
K+
|
821
|
715
|
682
|
649
|
Rb+
|
785
|
689
|
660
|
630
|
Cs+
|
740
|
659
|
631
|
604
|
Lattice Energies of Salts of F- (kJ mol-1)
| |
F-
|
Na+
|
923
|
Mg2+
|
2957
|
Al3+
|
5492
|
The lattice energy is the energy liberated when oppositely charged ions in
the gas phase come together to form a solid. So for sodium chloride the lattice
energy is 787 kJ mol–1. This is the energy liberated when Na+
and Cl– ions in the gas phase come together to form the lattice
of alternating Na+ and Cl– ions in a NaCl crystal.
The charges and sizes of the ions are the critical factors which determine
the magnitude of the lattice energy. The lattice energy is related to the potential
energy of two interacting charges which is given in the mathematical equation;
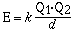
Q1 and Q2 are the magnitudes of the charge on the particles
in coulombs and d is the distance between centers in meters. The constant k has a value of 8.99 x 109J m C–2. From this relationship
the magnitude of the lattice energy is directly related to the charge on the
ions and inversely related to the ionic radii of the ions. The combination of
a small anion and a small cation liberates more energy compared to the combination
of large ions. In general holding one of the ions constant the trend in lattice
energy is reflected in the change in size of the counterion.
The strength of the ionic bond also depends on the magnitude of the charge on
the cation or anion. In the case of the fluoride compounds of sodium, magnesium
and aluminum there is a dramatic difference in the lattice
energy with cation charge.
It is the formation of the ionic bond which is the driving force for this
reaction. In the solid sodium chloride considerable energy is liberated as a
result of the arrangement of the sodium and chloride ions.
To generalize this electron lose and electron gain behavior we should note
that Group IA metals, with their single valence electron will easily lose the
electron to form a +1 cation. A behavior that continues down the Group. Group
IIA metals will lose their two valence electrons to form M2+ cations.
When this occurs the cations are isoelectronic with the preceeding noble gas.
Group IIIA metals form M3+ species, although as we go down the Group
we do see M+ species appearing. Removing 3 electrons requires considerably
more energy, and as a result we see fewer ionic compounds in Group IIIA compared
to Group IA and Group IIA.
Group IVA and VA exhibit little tendency to lose or gain electrons to form
ionic bonds. Members of these two groups prefer to share electrons.
In Groups VIA and VIIA, elements with high electron affinities, we see gain
of electrons to become isoelectronic with the next noble gas. Group VIA gaining
two electrons and Group VIIA gaining one electron.