Covalent Compounds
As important and varied as ionic compounds are, there are many compounds which
do not demonstrate the high melting points, high water solubility and electrical
conductivity of ionic compounds. This other group of compounds consists of examples
that are gaseous, liquids and solids at room temperature, and generally they
have low melting or boiling points. Frequently they are insoluble in water.
They do not conduct electricity in the liquid state, or when soluble in water,
do not conduct electricity in aqueous solution. Compounds that do not conduct
electricity are called nonelectrolytes. There is no evidence to indicate
that the elements in these compounds are ionic. Rather there is considerable
experimental evidence to suggest that such compounds are made of discrete molecules,
hence compounds in this group are called molecular compounds.
The forces that hold the atoms together in molecular compounds can not be
understood on the basis of oppositely charged ions. So it was that Gilbert Lewis
proposed that the strong attractive force between two atoms in a molecule reselted
from a covalent bond, formed by sharing of a pair of electrons
between the atoms in the bond.
In hydrogen, H2, as the two hydrogen atoms approach one another
their spherical 1s orbitals begin to overlap.
Each electron occupying the space around the two nuclei. Each electron is attracted
simultaneously by each nuclei. The attraction that bonds the electrons to both
nuclei is the force holding the atoms together. Thus while ions do not exist
in covalent compounds the bond can be regarded as arising from the attraction
of oppositely charged particles–nuclei and electrons. Covalent bonds can be describe azs resulting from the overlap of atomic orbitals resulting in the sharing of electrons in the covalent bond.
We can represent the formation of the covalent bond in hydrogen by writing
the Lewis electron–dot formula for the atoms and the molecule.
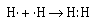
The two electrons between the two hydrogen nuclei represent a covalent
bond. And the two electrons are referred to as an electron pair.
Each hydrogen atom can be thought of as sharing the pair of electrons. When
we think in these terms we note that each atom has a 1s2 electron
configuration, isoelectronic with the next noble gas–helium.
The formation of the covalent bond in gaseous HF can be described in similar
terms;
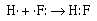
The fluorine atom and the hydrogen atom each require one electron to achieve
a configuration isoelectronic with a noble gas. Helium for hydrogen and neon
for fluorine. The two atoms share the electron pair to achieve their respective
noble gas configurations. The other three pairs of electrons around the fluorine
atom are called nonbonding or lone pair electrons. Review this movie to represent the covalent bond that forms between hydrogen and fluorine in HF.
We can understand the formulas of a large number of covalent compounds by
writing or drawing the Lewis electron–dot formulas for the compounds.
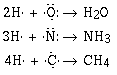
(Use lines to denote the covalent bond and dots to denote the electron
pairs.)
Look at a movie modeling the overlap of atomic orbitals in water, H2O and a movie modeling the overlap of atomic orbitals in ammonia, NH3.
terms of the atomic orbitals on the atoms. Notice that in writing our formulas
that F, N, O and C all have eight electrons around it. The tendency of atoms
in a molecule to share electrons to have a total of eight is called the octet
rule. Many compounds follow the octet rule, and some do not.
So far all the examples we've discussed involved atoms sharing two electrons.
Two electron bonds are called single bonds. However, it is possible for
two atoms to share two pairs of electrons to form double bonds and even
three pairs of electrons to form triple bonds. Atoms such as C, N, O
and S form double bonds in certain instances, while C and N exhibt examples
of triple bonds.
Although the covalent bonds in dihydrogen, H2, and hydrogen
fluoride, HF, each involve a pair of electrons being shared, there are some
important differences in the way inwhich the electrons are shared. In hydrogen
it can be noted that the electrons spend an equal amount of time near each nucleus.
However, it can not be said that the electron pair shared between hydrogen and
fluorine in HF is equally shared. In fact, the electrons spend more time near
the fluorine nucleus than near the hydrogen nucleus. Resulting in the fluorine
appearing to have some extra negative charge and the hydrogen some extra positive
charge.

The covalent bond in HF is described as a polar covalent bond because
the electrons spend an unequal amount of time on the two nuclei. The bond in
hydrogen is called a nonpolar bond. It should be noted that the polar
bond can be viewed as an intermediate case between the equal sharing of electrons
in hydrogen and the transfer of electrons that arises in ionic bonds.
In general, atoms form compounds by losing, gaining or sharing enough electrons to
achieve the outer electron configuration of a noble gas. The combining capacities
of atoms are a consequence of the proportions in which they must associate to
achieve noble gas configurations.
ELECTRONEGATIVITY
Electronegativity is a concept which is used to help understand which
atom in a covalent bond will have the greatest attraction for the bonding pair
of electrons. Electronegativity is a measure of the ability of an atom in a
covalent bond to attract electrons to itself.
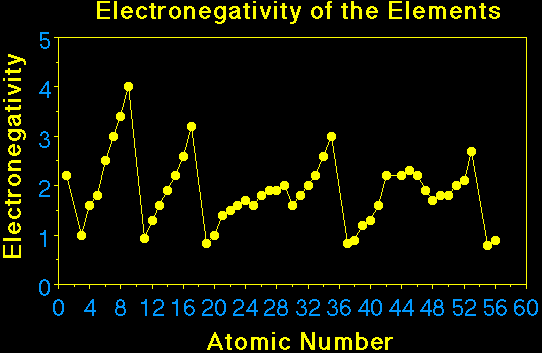
From the figure one can see the important trend in electronegativity across
a period and down a group. In general the electronegativity increases going
across a period, and decreases going down a group. The larger the electronegativity
value the greater the attraction of the electron by the atom. The absolute value
of the difference in electronegativity of two atoms sharing a pair of electrons
is a measure of the degree of polarity of the chemical bond. When the difference
is small the bond is nonpolar. When it is large the bond is polar, if the difference
is very large the bond is ionic.
<