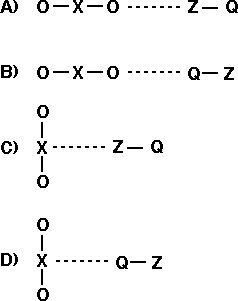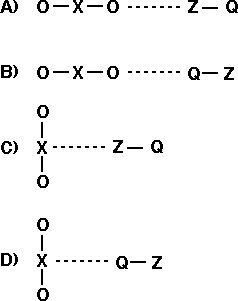Clicker Questions to be used during classroom discussion and DCI activity for introduction to chemical kinetics.
1. Which of the following best represents the balanced chemical equation for the reaction in the today's BCE?
A. G + B --> BG
B. G + 2B --> GB2
C. G2 + B ---> G2B
D. 2G ---> G2
E. 2G + B ---> G2B
F. G + GB --->G2B
G. 8G + 4B ---> 4G2B
2. Which of the following collisions result in the formation G2B?
A. G + B
B. 2G + B
C. G2 + B
D. BG + G
E. BG + G2
F. G + G
3. What collision(s) is(are) required to convert reactants to products:
A. G + G and G2 + B
B. G + G + B
C. GB + B
D. B + B and G + G and G2 + B2
E. B + G and BG + G
4. Which of the following is the best mechanism to describe the reaction 2G + B --> G2B.
A. Step 1: G + G + B ---> G2B
B. Step 1: G + B ---> GB / Step 2: GB + G ---> G2B
C. Step 1: B + B ---> B2 / Step 2: G + G ---> G2 /Step 3: B2 + G2 ---> G2B + B
D. Step 1: G + G ---> G2 / Step 2: G2 + B ---> G2B
E. Step 1: B + G ---> BG / Step 2: G + G ---> G2 /Step 3: BG + G2 ---> G2B + G
5. Given the following mechanism
Step 1: NO2 ---> NO + O
Step 2: O + NO2 ---> O2 + NO
Write the overall reaction given a proposed mechanism for the reaction.
A. NO2 --> NO + O
B. NO2 --> N + O2
C. 2NO2 --> 2NO + O2
D. 2NO2 + O --> 2NO + 3/2O2
E. NO2 + O ---> NO + O2
6. Identify the intermediate(s) in the mechanism for Question 5
A. NO and O
B. O2
C. NO
D. O
E. NO and O2
F. NO3
7. A reasonable mechanism for the reaction
NO2 + CO ---> NO + CO2
is
A. Step 1: NO2 + CO ---> NO + CO2
B. Step 1: 2NO2 ---> NO + NO3 / Step 2: NO3 + CO ---> NO + CO2
C. Step 1: NO2 + NO2 + CO ---> CO2 + NO3 / Step 2: NO3 ---> NO + O
D. Both A and B
E. Both B and C
F. A, B and C
8. What reason(s) account for the fact that not all gas phase collisions are successful in producing a chemical change?
I. the orientation of the reacting particles is not geometrically optimal;
II. the particles that collide do not have sufficient energy (are not moving fast enough);
III. the volume of the container is too large to allow a sufficient number of collisions to occur;
A) I only
B) II only
C) III only
D) I and II
E) II and III
F) I and III
E) I, II and III
9. Given the reaction: XO2(g) + ZQ(g) ---> XO(g) + OZQ(g)
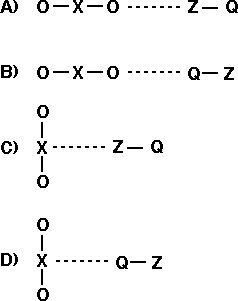
Teaching Notes:
Clicker Question 1 looks for the student group responses from Question 1 on the DCI.
Clicker Question 2 looks for a slightly different response to the Question 2 on the DCI.
Clicker Question 7 will lead to the importance of experimental data for supporting a proposed mechanism.
Clicker Questions 4, 5 and 7 lead to the idea that a balanced chemical equation does not provide all of the possible information about what is happening in a chemical reaction.
Clicker Questions 8 and 9 point out the importance of orientation in effective collisions.