a) atom
An atom is the smallest unit of matter. It is composed of a nucleus (that contains protons and neutrons) where most of the mass is located and electrons that have a very small mass and are located outside of the nucleus. If we are to imagine an atom in our minds a spherical shape is reasonable.
b) molecule
A molecule is also a small unit of matter. It is composed of two or more nonmetallic atoms that are 'joined' together by a covalent bond.
c) element
An element is a term used to describe matter in a pure form, that contains only one kind of atom. An example of an element is carbon, oxygen, or chlorine. Water, cardboard, or potatoes would not be an example of an element.
d) compound
A compound is a term used to describe matter in a pure form, that contains matter composed of two or more elements chemically combined in a fixed ratio. Substances like water, carbon dioxide, sodium chloride and sugar are examples of compounds. Carboard, potatoes, or an ant would not be examples of compounds.
e) formula
A symbolic way to represent pure matter, such as an element or a compound. The formula for the element oxygen is O2. The formula for the compound carbon dioxide is, CO2.
f) solution
A solution is an example of matter that is not pure, but contains a homogeneous mixture of more than one type of element or compound. A homogeneous mixture is uniform throughout. All components of a homogeneous mixture appear to be in the same phase. Finally, it is relatively easy to separate the components of a solution back into their original pure state. An example of a solution would be sodium chloride dissolved in water. Another example of a solution is the atmosphere. Milk or orange juice would not be examples of a solution.
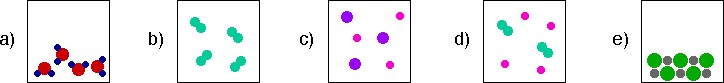
2. Here are five boxes that contain matter depicted at the particulate level (level of atoms and molecules).
Use the boxes above to answer each of the following questions:
i) Which of the box(es) represents a sample of an element? Select all that apply. (Note: look at your definition above to help you decide.)
Figure a is a compound not an element because each molecule contains a red element and two black elements. Figure b is an element, that exists as diatomic molecules. Figure c is a homogeneous mixture of two different monoatomic elements. Figure d is a homogeneous mixture of two different elements, a monoatomic element and a diatomic element. Figure e is an example of an ionic compound, composed of a small cation and a larger anion.
ii) Which of the box(es) represents a sample of a compound? Select all that apply. (Note: look at your definition above to help you decide.)
Figure a is a compound because each molecule contains a red element and two black elements. Figure b is an element, that exists as diatomic molecules. Figure c is a homogeneous mixture of two different monoatomic elements. Figure d is a homogeneous mixture of two different elements, a monoatomic element and a diatomic element. Figure e is an example of an ionic compound, composed of a small cation and a larger anion.
iii) Which of the box(es) represents a sample of a pure substance in the liquid phase? Select all that apply.
Figure a is a pure substance (compound) in the liquid phase. Figure b is a pure substance (element) in the gas phase. Figure c is a homogeneous mixture of two different monoatomic elements in the gas phase. Figure d is a homogeneous mixture of two different elements, a monoatomic element and a diatomic element in the gas phase. Figure e is is a pure substance (compound) in the solid phase.
iv) Which of the boxes represents a sample of a solution? Select all that apply.
Figure a is a pure substance (compound) in the liquid phase. Figure b is a pure substance (element) in the gas phase. Figure c is a solution (homogeneous mixture) of two different monoatomic elements in the gas phase. Figure d is a solution (homogeneous mixture) of two different elements, a monoatomic element and a diatomic element in the gas phase. Figure e is is a pure substance (compound) in the solid phase.
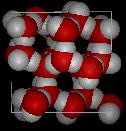
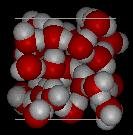
3. Below are two images representing a sample of water in two different phases at the level of atoms and molecules (the particulate level).
Figure I. |
Figure II. |
a) Assuming the volume of the two figures is the same, which Figure has the lowest density.
Explain how you arrived at your answer.
Figure I depicting the solid phase of this substance would have the lowest density. Since both figures have the same volume and the same type of matter, there appears to be less matter in Figure I compared to Figure II. Density is defined the mass/volume. If volume is the same, the volume with the smallest mass of matter will have the smallest/lowest density.
b) Identify the phase (gas, liquid or solid) depicted in Figure I.
The substance in Figure I is shown in the solid phase. Note how organized the particles are in the figure. Also the particles are very close together. There does appear to be 'hole' which is an interesting observations. More about that in class.
c) Identify the phase depicted in Figure II.
The substance in Figure II is shown in the liquid phase. Note how disorganized the particles are in the figure. Also the particles are very close together.
d) Provide the symbol of the element for each type of atom in the figure.
Atom |
Symbol |
|---|---|
Red Atom |
O (oxygen) |
White Atom |
H (hydrogen) |