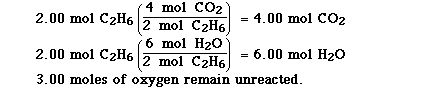A problem which is more fun involves specifying the amounts
of both reactants without indicating which is in excess. It
becomes necessary to determine which of the reactants is in
excess.
Lets begin with a simple example to show the logic. In the
reaction,
2C2H6(g) + 7O2(g)
---> 4CO2(g) + 6H2O (g)
Exactly 2 mols of C2H6 are required to
react completely with 7 mols of O2.
Sample Limiting Reagent Problem #1
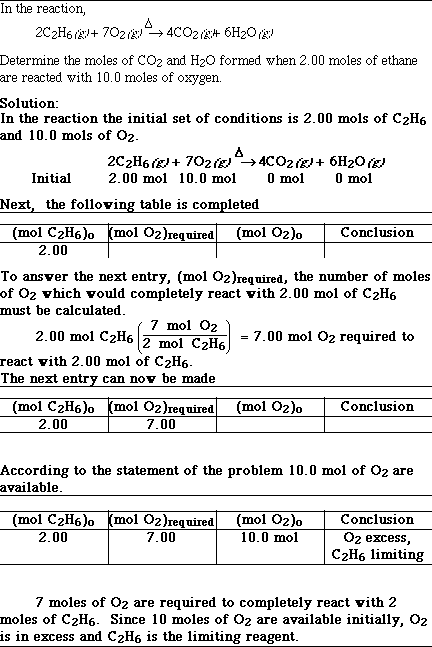
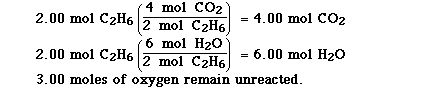
Sample Limiting Reagent Problem #2

Sample Limiting Reagent Problem #3

Here are two animations showing the second problem using two
different approaches. One as we solved the problem in class the
other a slightly different way.