What is a chemical bond, or just plain
bond?
The generally accepted definition of a bond is a
force (some type of interaction) which holds atoms
together so the bonded atoms can function as a unit as
described by the chemical formula of the substance.
We usually describe a bond as a force because we know it
takes energy to separated atoms that are bonded together. The
energy required to separate the atoms depends on the atoms that
are bonded together.
So what is the force which holds the atoms together? It is a
very fundamental force, but it manifests itself in slightly
different ways. Fundamentally the force is attractive and the
attraction results from the opposite charge of the electron and
the nucleus. How the attraction of opposite charges manifest
itself in a chemical bond depends on whether the electrons are
transferred or shared by the two atoms forming the bond.
How do we know whether an atom wants to
transfer or share its electrons?
Metals love to lose electrons!
So when a metallic atom interacts with a nonmetallic atom
electron transfer occurs. But when two nonmetallic atoms interact
electrons are shared. It is pretty much that simple!
How many electrons does a metal want to
lose?
A metal in Group IA, IIA or IIIA will lose as many
electrons as are required to attain the electron
configuration of the nearest noble gas element (Group
VIIIA).
For example, sodium has an electron configuration of 1s22s22p63s1.
Notice sodium has one valence electron because of the single
electron in its outer most shell. By losing that valence electron
the electron configuration becomes 1s22s22p6,
which is the same electron configuration for neon (the nearest
noble gas). Sodium is in Group IA. All of the metallic elements
in Group IA lose one electron. Metals in Group IIA lose two
electrons, and metals in Group IIIA lose three electrons. When a
metal loses electron (negatively charged) the species left has a
positive charge.
An important point about the transition metals: in general
they like to lose two or three electrons.
Who gains the electrons lost by the
metallic element?
How many electrons will a nonmetallic
element gain?
A nonmetal in Group VIIA, VIA or VA will gain as many
electrons as are required to attain the electron
configuration of the nearest noble gas element (Group
VIIIA).
For example, chlorine has an electron configuration of 1s22s22p63s23p5.
Notice chlorine has seven valence electron because of the seven
electrons in its outer most shell. By gaining one valence
electron the electron configuration becomes 1s22s22p63s23p6,
which is the same electron configuration for argon (the nearest
noble gas). Chlorine is in Group VIIA. All of the nonmetallic
elements in Group VIIA gain one electron. Nonmetals in Group VIA
gain two electrons, and nonmetals in Group VA gain three
electrons. When a nonmetal gains an electron (negatively charged)
the species has a negative charge.
When a cation (positively charged metallic ion) interacts
with an anion (negatively charged ion) an ionic bond is formed.
An ionic bond occurs when the force is electrostatic (due to
oppositely charged ions) in nature. Ions are formed when
electrons are transferred between a metallic element and a
nonmetallic element.
Predict the formula for some ionic compounds. In class we
discussed two examples;
Determine the formula of the compound formed from Na and
Cl2. To solve this problem we recognized one of
the elements is a metal. Metals like to lose electrons. How
many electrons will sodium lose? Since it is in Group IA, it
has one valence electron and it will lose that one electron,
forming Na+. Chlorine, on the other hand, is a
nonmetal. In the presence of a metal, nonmetals gain
electrons. How many electrons will chlorine gain? It is in
Group VIIA so it will gain one electron, forming, Cl-.
To write the formula we determine how many of each ion is
required to balance the charge, or have a total charge of
zero. In this case the formula must be NaCl. (Note: Even
though the standard state of chlorine is a diatomic molecule,
Cl2, when we write formulas and consider the
number of electron gained or lost by an element we consider
the element in terms of a single atom. When write a chemical
equation to describe the reaction between sodium and chlorine
and the product formed balancing the equation resolves the
total number of atoms issue.)
Determine the formula of the compound formed from Na and
O2. To solve this problem we recognized one of the
elements is a metal. Metals like to lose electrons. How many
electrons will sodium lose? Since it is in Group IA, it has
one valence electron and it will lose that one electron,
forming Na+. Oxygen, on the other hand, is a
nonmetal. In the presence of a metal, nonmetals gain
electrons. How many electrons will oxygen gain? It is in
Group VIA so it will gain two electron, forming, O2-.
To write the formula we determine how many of each ion is
required to balance the charge, or have a total charge of
zero. In this case the formula must be Na2O.
(Note: Even though the standard state of chlorine is a
diatomic molecule, O2, when we write formulas and
consider the number of electron gained or lost by an element
we consider the element in terms of a single atom. When write
a chemical equation to describe the reaction between sodium
and chlorine and the product formed balancing the equation
resolves the total number of atoms issue.)
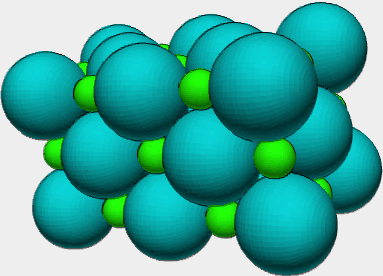
So now we need to spend a minute thinking about what an ionic
compound 'looks like' when viewed at the atomic level. Since
ionic compounds are composed of ions, and when we think of an ion
the best shape to imagine is a sphere. Anions are usually larger
spheres than cations, but both are spheres. The sphere is the
best shape because ions have filled electronic shells. So the
best way to think about the arrangement of the ions at the atomic
level is in three dimensional arrays where each ion is surrounded
by the ion of opposite charge. Here is an example of a small
section of the arrangement common for alkali halides like NaCl.