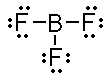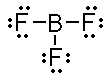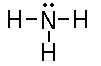If we do the Lewis structures for both compounds we have;
BF3
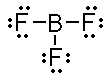
NH3
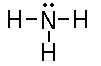
An interesting observation is that BF3 does follow
the octet rule...ANOTHER EXCEPTION! In Group IIIA boron does not
always follow the octet rule. In some of its chemistry it is
'happy' with three pairs of electrons. So who are we to argue. SO
what would the molecular geometry be for BF3? Since it
has no lone pairs and three bonding groups on the central atom it
is trigonal planar. Since all the terminal atoms are identical
and there are no lone pair on the central atom BF3 is
nonpolar.
How about NH3? In this case the molecular geometry
is trigonal pyramidal. The central nitrogen atom has a lone pair
of electrons on it, therefore is NH3 polar.