![]()
heat and light (flames)
explosion
color change
evolution of a gas
formation of a solid (precipitate)
heat (using a delta symbol)
temperature (at which the reaction is run at)
pressure
time (length of time the reaction is allowed to proceed)
Fe(s) + S8(s) --heat--> FeS(s)
S8(s) + O2(g) --heat--> SO2(g)
Na(s) + Cl2(g) ----> NaCl(s)
N2(s) + O2(g) --heat--> 2NO2(g)
C(s) + O2(g) ----> CO2(g)
P4(s) + 5O2(g) --heat--> P4O10(s)
Balance the following equations;a) S8(s) + O2(g) --heat--> SO2(g) Answerb) Fe3O4(s) + H2(g) ----> Fe(s) + H2O(l) Answer |
Name |
Formula |
methane |
CH4(g) |
ethane |
C2H6(g) |
propane |
C3H8(g) |
butane |
C4H10(g) |
pentane |
C5H12(l) |
hexane |
C6H14(l) |
heptane |
C7H16(l) |
octane |
C8H18(l) |
nonane |
C9H20(l) |
decane |
C10H22(l) |
Balance the following equations;a) CH4(g) + O2(g) --heat--> CO2(g) + H2O(g) Answerb) C4H9SH(g) + O2(g) --heat--> CO2(g) + H2O(g) + SO2(g) Answerc) C4H10(g) + O2(g) ----> CO2(g) + H2O(l) Answer |
|
|
To begin we looked at the conductivity of a sample of deionized water (water with little or no ions) which could be viewed as pure water. Looking at the image on the left the light bulb is not glowing. This can be explained in terms of the nature of the species in the sample of water. That the light does not glow suggests there are no ions in the sample. This is an important measurement to establish subsequent measurements in deionized water. Any observed conductivity will due to the presence of the solute. |
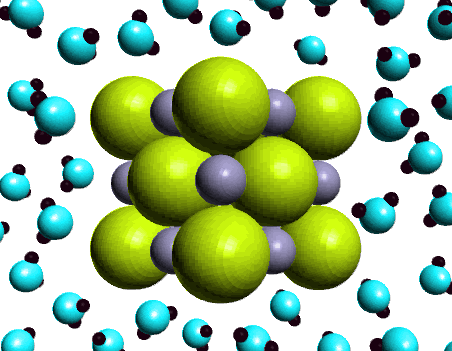
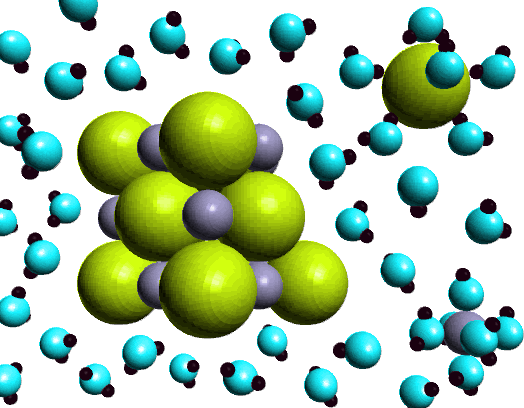
Next we measured the conductivity of an aqueous solution of sodium chloride. Here the light bulb glows brightly indicating the presence of ions in the solution. The ability of an aqueous solution of sodium chloride to conduct electricity is characteristic of a strong electrolyte. How do we express this observation using a chemical equation?NaCl(s) ---H2O--> Na+(aq) + Cl-(aq)Soluble ionic compounds are also strong electrolytes, i.e., behave the same way as sodium chloride...forming ions when dissolved in water. How do we know whether an ionic compound dissolves in water? That is determined by experiment. A Solubility Table summarizes the experimental observations of the solubility behavior of a large group of ionic compounds. We'll discuss a Solubility Table shortly. |
|
|
|
When we tested the conductivity of an aqueous solution of sucrose the light bulb did not glow. This type of behavior is characteristic of a nonelectrolyte. No ions in this solution, yet the sucrose dissolved. How do we write a chemical equation in this case?C12H22O11(s) ---H2O--> C12H22O11(aq)In general soluble covalent compounds do not dissociate into ions when dissolved in water. |

Name |
Formula |
Sulfuric acid |
H2SO4 |
Sulfurous acid |
H2SO3 |
Nitric acid |
HNO3 |
Nitrous acid |
HNO2 |
Phosphoric acid |
H3PO4 |
Phosphorus acid |
H3PO3 |
Carbonic acid |
H2CO3 |
Perchloric acid |
HClO4 |
Acetic acid |
HC2H3O2 |
Formula |
Name |
HF(aq) |
Hydrofluoric acid |
HCl(aq) |
Hydrochloric acid |
HBr(aq) |
Hydrobromic acid |
HI(aq) |
Hydroiodic acid |
H2S(aq) |
Hydrosulfuric acid |
HCN(aq) |
Hydrocyanic acid |
Name of Base |
Formula of Base |
Sodium hydroxide |
NaOH |
Potassium hydroxide |
KOH |
Barium hydroxide |
Ba(OH)2 |
Ammonia |
NH3 |
Calcium hydroxide |
Ca(OH)2 |
Aluminum hydroxide |
Al(OH)3 |
2Li(s) + 2H2O(l) ---> 2LiOH(aq) + H2(g)
2Na(s) + 2H2O(l) ---> 2NaOH(aq) + H2(g)
2K(s) + 2H2O(l) ---> 2KOH(aq) + H2(g)
Mg(s) + 2HCl(aq) ---> MgCl2(aq) + H2(g)