Predict whether 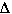 S
is positive, negative, or close to zero for the following changes.
S
is positive, negative, or close to zero for the following changes.
d) CH4(g) + 3O2(g) ----> CO2(g)
+ 2H2O(l)
Answer
In this example 4 moles of reactants form 3moles of products.
Also the reactants are all gases and the in the products there is one mole of
gas and two moles of liquid. This is a case where the products are more ordered
than the reactants, and 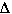 S is negative.
S is negative.