Using the vapor pressure data for acetic acid, CH3COOH(l),
|
Temperature (degrees C)
|
Vapor Pressure (mmHg)
|
10.0
|
6.00
|
20.0
|
11.6
|
30.0
|
21.3
|
40.0
|
37.3
|
50.0
|
63.7
|
Here the table is completed.
T (degrees C)
|
T (Kelvins)
|
T-1
|
Pv (mm Hg)
|
ln (Pv)
|
10.0
|
283
|
0.00353
|
6.00
|
1.79
|
20.0
|
293
|
0.00341
|
11.6
|
2.45
|
30.0
|
303
|
0.00330
|
21.3
|
3.06
|
40.0
|
313
|
0.00319
|
37.3
|
3.62
|
50.0
|
323
|
0.00310
|
63.7
|
4.15
|
Now plot the data with ln (Pv) on y-axis and on the T-1
x-axis. After you have plotted the data go to the next page to see the plot.
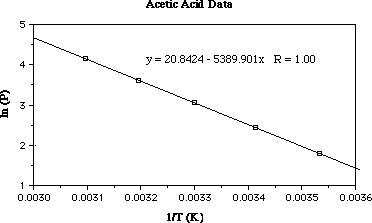
In the plot above I've plotted the data (ln (Pv) versus T-1).
I also did a linear regression on the data to get the equation for the line
(the R value is perfect...great professor data!).
Can do determine the delta H of vaporization for ammonia?
Answer;
From the slope of the line in the plot we recall;
slope = ÐÆHovap/R
From the linear regression the slope of the date is equal to Ð5390 K-1;
Substituting;
Ð5390 K-1 = ÐÆHovap/R
In this equation R has a value of 8.314 J mol-1 K-1
ÐÆHovap = Ð5390 K-1 x 8.314
J mol-1 K-1
ÐÆHovap = 44.8 kJmol
Now answer the following two questions;
Use the your plot to determine the temperature at which acetic
acid has a vapor pressure of 42.4 mmHg.
Use the your plot to determine the vapor pressure of acetic acid
at 13.5 ûC.
Answers to these last two questions.
Here is the data plotted again (like the plot above) only I've added two lines
to interpolate the plot to answer the two questions.
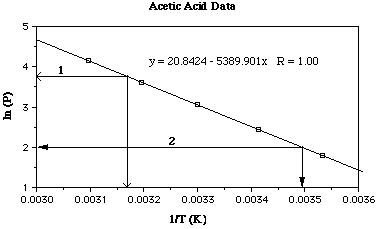
Use the your plot to determine the temperature at which acetic
acid has a vapor pressure of 42.4 mmHg.
Answer:
From the graph above line '1' intersects the y-axis at 3.75 (ln 42.4). Dropping
a perpendicular line to the x-axis intersects at 0.00317.
So
T-1 = 0.00317
T = 315 K
Use the your plot to determine the vapor pressure of acetic acid
at 13.5 ûC.
Answer:
From the graph above line '2' intersects the x-axis at T-1
= 0.00349, 286.5 K. Drawing a perpendicular line to the y-axis intersects at
ln 2. Or P = 7.39 mmHg.