Choose the member of each set that has the higher solubility
in water. Briefly, explain your answer.
b) Carbon dioxide or silicon dioxide
Answer:
To begin to answer this question we need to determine the most
important intermolecular attractive force for both of these compounds. To assign
the IMAF we need to know if the compound is polar or nonpolar.
So first determine the polarity of carbon dioxide and silicon
dioxide.
The Lewis structure for carbon dioxide is;

The ball-and-stick model of carbon dioxide is;

This molecule is nonpolar and the only forces are dispersion
forces.
Now we will look at silicon dioxide. Silicon dioxide is a solid
that belongs to the class we labeled as extended covalent. That is, not only
are the oxygen atoms in an SiO2 group bonded to the silicon, but in the extended
covalent structure a 'red' oxygen atom is bonded to another blue silicon atom
and that atom is covalently bonded to another oxygen atom. So we can trace through
the all the Si-O bonds to see all the atoms form a large macromolecule where
each oxygen atom is bonded to two silicon atoms and each silicon.
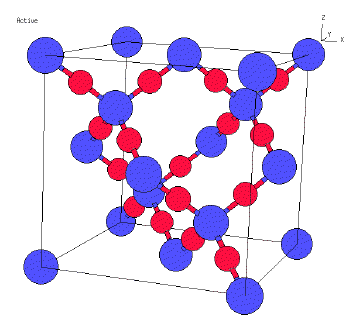
So the attractive forces are all covalent bonds in silicon dioxide.
With such strong forces, silicon dioxide will not dissolve in water.
So now we have two substance neither of which is predicted to
be soluble in water...what do we do. We'll lets think about these two substances
from our common experience. Carbon dioxide is a gas and even though it is nonpolar
we know that soft drinks have dissolved carbon dioxide. Silicon dioxide is the
major componenet of sand and we know sand does not dissolve in water.
So we have to conclude that even though both are predicted to
be insoluble in water, one of the species, carbon dioxide, does have some slight
solubility. This brings up an important point about solubility. Solubility is
a measure of the extent a solute will dissolve in a solvent. Even a substance
labeled as insoluble in water will dissolve to some amount. This is the case
for carbon dioxide. However, the extremely strong covalent bonds in silicon
dioxide can not be broken down by water and it is definitely insoluble in water.