Some atoms have a greater attraction for electrons than others. The concept of electronegativity uses the attraction for electrons by atoms to understand the ability of atoms sharing electrons in chemical bonds to attract electrons. A number can be assigned to atoms to represent the atom's relative tendency to attract valence electrons. This number is the Electronegativity value of an atom. Below is a Table of Electronegativity values for some of the atoms in the periodic table. We also need to remember that it is the valence electrons in atoms that are involved with form chemical bonds.
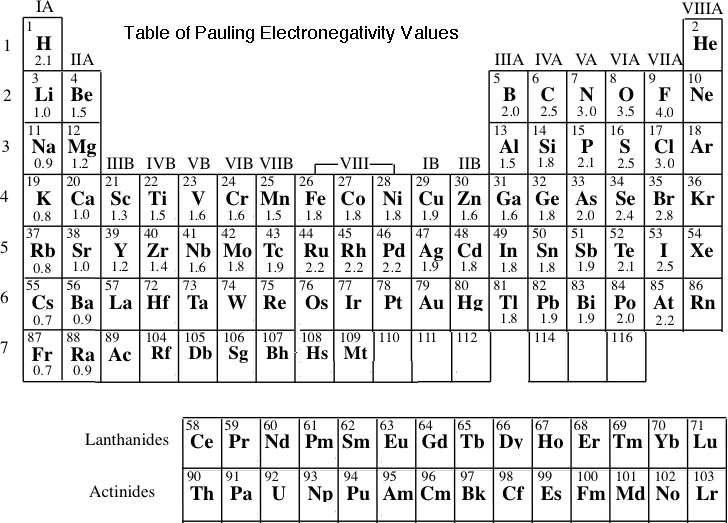
To better understand this table answer the following questions.
1. Enter the symbol for the element with the largest electronegativity value.
From the table of electronegativity values, fluorine has the largest value of 4.0.
2. Based on the value of the electronegativity you selected for Q1, select all the apply from the following statement's about this element and its electronegativity value.
a) this element does not attract valence electrons from other atoms;
b) this element attracts its own valence electrons;
c) this element attracts valence electrons of other atoms bonded to it;
d) this element has the greatest attraction to valence electrons compared to any other element in the periodic table;
e) this element has the smallest atttraction to valence electrons of any other element in the periodic table.
Fluorine has the highest electronegativity value can be interpreted to mean that; fluorine (c) attracts valence electrons of other atoms bonded to it, and since the EN is the largest, fluorine will have (d) the greatest attraction to valence electrons compared to any other element in the periodic table
3. Going across a period (row) from left to right in the periodic table the general trend in electronegativity values is;
a) increase;
b) decrease;
c) remains constant.
Going across a period (row) from left to right in the periodic table the general trend in electronegativity values is to increase. If we look at the fourth period there are instances where this does not happen as we step from let to right, however, overall the trend in electronegativity values across a period is to increase.
4. Going down a group (column) from top to bottom in the periodic table the general trend in electronegativity values is;
a) increase;
b) decrease;
c) remains constant.
Going down a group (column) from top to bottom in the periodic table the general trend in electronegativity values is to decrease.
5. Look at each substance in the table below and complete the missing information. Indicate whether the compound is ionic or covalent based on all of our class discussions to date; then use the periodic table above to find the electronegativity value for each element, and then calculate the difference by always subtracting the smallest electronegativity value from the largest. NOTE: Values for the first substance, NaCl, have been entered as an example.
Table II.
Substance |
Ionic/covalent |
EN value |
EN Value |
|
|---|---|---|---|---|
NaCl |
ionic |
Na 0.9 |
Cl 3.0 |
2.1 |
KBr |
|
K
|
Br
|
|
MgO |
|
Mg
|
O
|
|
HCl |
|
H
|
Cl
|
|
HF |
|
H
|
F
|
|
O2 |
|
O
|
O
|
|
H2 |
|
H
|
H
|
|
6. Looking at the Table of Electronegativity values at the beginning of the BCE, what is the formula of a compound that would have the largest  EN.
EN.
The formula would be FrF (francium fluoride). The EN value for Fr is 0.7 and for fluorine the EN value is 4.0 so  EN is 3.3.
EN is 3.3.
7. Is the compound you identified in Q6 ionic or covalent?
FrF would be ionic. Francium's EN is very small so the valence electron is easily transferred to fluorine, which has a very large EN.
8. Using the data in Q5 - Q7 which of the following statements describes the relationshiop between bonding types and  EN. (choose all that apply)
EN. (choose all that apply)
a) there is no relationship;
b) covalent bonds must have a  EN equal to 0;
EN equal to 0;
c)
ionic bonds must have a  EN between 3.3 and 2.0;
EN between 3.3 and 2.0;
d) covalent bonds must have a  EN less than 2.0;
EN less than 2.0;
The relationship between bonding types is that (c) ionic bonds must have a  EN between 3.3 and 2.0 and (d) covalent bonds must have a
EN between 3.3 and 2.0 and (d) covalent bonds must have a  EN less than 2.0.
EN less than 2.0.
9. Choose from the following statements all that would apply to describing a compound containing a covalent bond between two atoms with a  EN equal 0?
EN equal 0?
a) the valence electrons in the covalent bond are shared equally between the two atoms;
b) the valence electrons in the covalent bond are shared unequally between the two atoms;
c) the two atoms bonded together are most likely identical;
When the  EN is zero between two elements that are covalently bonded then (b) the valence electrons must be shared equally between the two atoms and (c) both elements must be the identical.
EN is zero between two elements that are covalently bonded then (b) the valence electrons must be shared equally between the two atoms and (c) both elements must be the identical.
10. Below is a figure depicting the distribution of electron density in the O2 molecule (on the left) and the HF molecule (on the right).


Which image (O2 or HF) do you believe represents a symmetrical distribution of electron density and which image (O2 or HF) do you believe represents an asymmetical distribution of electron density.
Symmetrical distribution
O2 has a symmetrical distribution of electron density between the two atoms.
Asymmetrical distribution
HF has an asymmetrical distribution of electron density between the two atoms.
11. In the image depicting HF what do you think the color blue and the color red represent? Select all that apply.
The blue color represent a region where there is little electron density, so that part of the molecule will have a partial positive charge. The red color represent a region where there is higher electron density, so that part of the molecule will have a partial negative charge.
12. In the diagram of HF below an arrow (vector) has been drawn. What do you think this arrow represents? Write a short explanation of what the arrow symbolizes in the diagram above: In your explanation use some or all of the following terms: covalent bond, polar covalent bond, nonpolar covalent bond, equal sharing of electrons, unequal sharing of electrons, electronegativity, difference in electronegativity, charge distribution, partial positive charge, partial negative charge, arrow/vector, dipole moment.
In the figure of HF the blue color around the hydrogen atom represents a region of low electron density, making the hydrogen atom in HF partially positive. The red color near the fluorine atom represents a region of high electron density, making the fluorine atom partially negative. The yellow 'arrow' represents a vector that indicates the unequal distribution of valence electrons that favors the more electronegative element, in this case fluorine. This arrow/vector is called the bond dipole. Notice that no such dipole exits in the O2 image. It is important to point out that the pointed end of the arrow points towards the atom in the bond with the higher electronegativity, and the opposite end of the arrow is located near the atom with the lower electronegativity.
13. Is there anything about the questions that you feel you do not understand? List your concerns/questions.
14. If there is one question you would like to have answered in lecture, what would that question be?