Thank you for completing this BCE. You may want to print this page out and bring it to class so if you have any questions you can ask them.
BCE Response Page
I recommend you print out this page and bring it to class. Click
here to show a set of five student responses, randomly selected from
all of the student responses thus far, in a new window.
, here are your responses
to the BCE and the Expert's response.
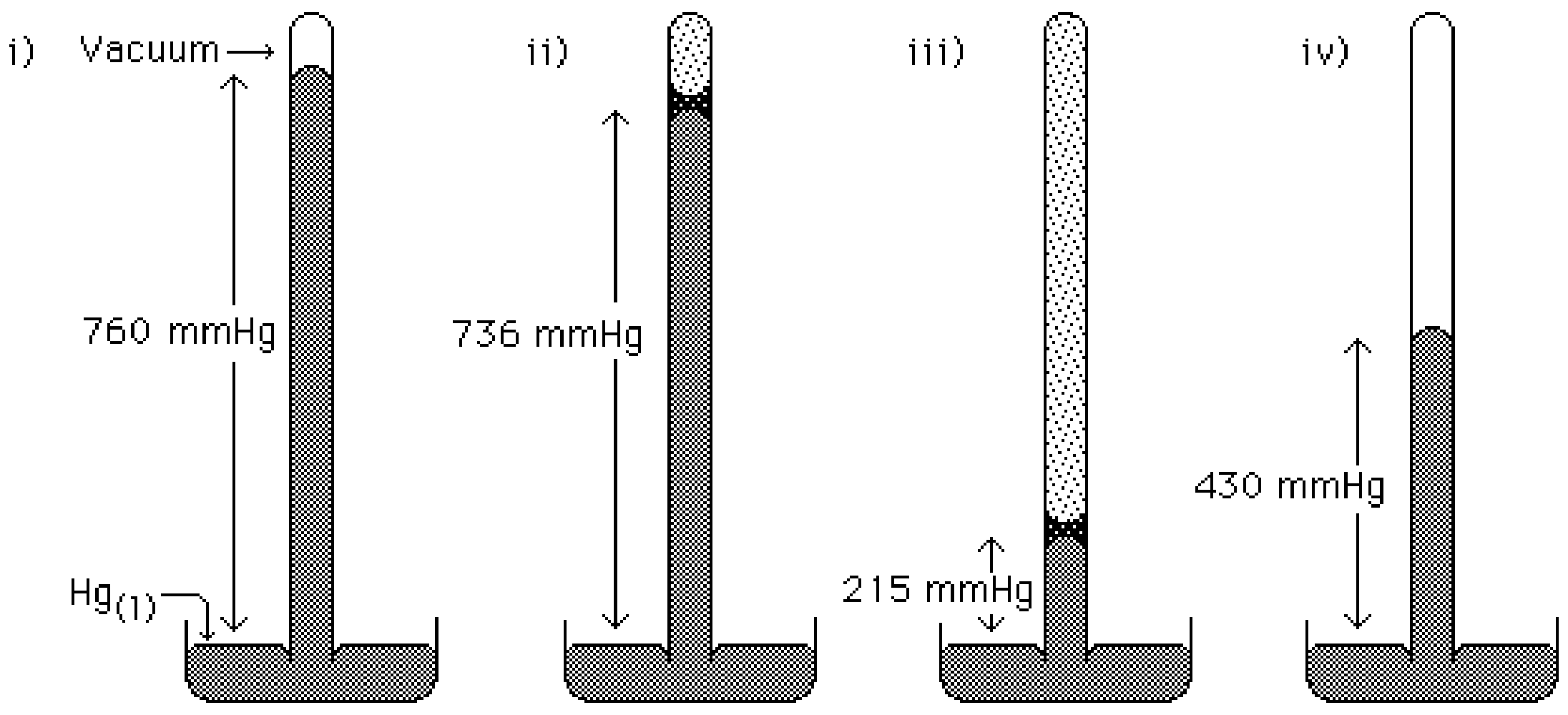
Consider the sketches of four barometers (above). Barometer i) shows the measurement of atmospheric pressure which is equivalent to the height the column of mercury is sustained (in this case atmospheric pressure is 760 mm Hg). Barometer ii) depicts the situation of a sample of water having been injected into the tube. Barometer iii) and iv) depict samples of diethyl ether having been injected into the tubes. All four barometers are at the same temperature.
1. Briefly describe/define vapor pressure for a liquid. (Check pages 425 and 426 in your textbook.)
The vapor pressure of a liquid is the pressure of the vapor above the liquid at a given temperature.
2. In barometer i) no liquid has been injected, so only mercury is present. Since the vapor pressure of mercury at 25 degrees Celsius is very low, it is assumed the small volume of space above the mercury liquid contains very few particles. In Barometer ii, a sample of water has been injected, why is the height of the mercury column lower in Barometer ii compared to Barometer i?
When a sample of a liquid is injected into a barometer (like i in the figure above), the sample liquid will immediately vaporize until the pressure exerted by the sample in the gas phase equals the vapor pressure for the liquid at that temperature. For example look at barometer ii, a sample of water was injected into the barometer (like i) and some of the water sample evaporated exerting a pressure. The water in the gas phase above the liquid water exerts a pressure of 24 mmHg at the temperature above. Since the water in the gas phase exerts a pressure the level of the mercury must drop an amount equal to the vapor pressure.
3. In the Figure above, what is the equilibrium vapor pressure of water?
mmHg
As describe above the vapor pressure of water can be determined by taking the difference between the pressure measure by the barometer without water, and the barometer with water injected. In this case the vapor pressure of water is 24 mmHg at the temperature above.
4. In the Figure above, what is the equilibrium vapor pressure of diethyl ether?
mmHg
545 mmHg is the vapor pressure of diethyl ether.
5. What would explain the height of the mercury column in Barometer iv? NOTE: A smaller sample of diethyl ether was injected into barometer iv compared to barometer iii.
In barometer iv a sample of diethyl ether has been injected, but all of the sample vaporized before the equilibrium vapor pressure was reached. To determine the equilibrium vapor pressure more diethyl ether must be injected into the barometer in iv. When that happens we will have the same case as shown in barometer iii. If we added even more diethyl ether to barometer iii, the vapor pressure of diethyl ether would not change, all that would happen is the volume of the liquid diethyl ether would increase, but the level of mercury would not change. This is an important point, the pressure exerted by the vapor of a liquid can never exceed the equilibrium vapor pressure. On the other hand it is possible to have a vapor pressure less than the equilibrium vapor pressure.

In the above figure is a plot of vapor pressure versus temperature for three liquids;
diethyl ether (red data points)
ethyl alcohol (blue data points)
water (black data points)
Using the graph answer the following questions;
6. Which liquid (diethyl ether, ethyl alcohol or water) has the highest vapor pressure at 25 °C?
According to the figure above the vapor pressure for diethyl ether is about 550 mmHg, the vapor pressure for ethyl alcohol is about 80 mmHg and the vapor pressure for water is about 24 mmHg at 25 °C.
7a. What is the normal boiling point of water?
°C
A normal boiling point is defined as the temperature at which the vapor pressure above the liquid equals 760 mmHg. It is a little difficult to tell in the figure above, but that temperature for water is 100 °C.
b) What is the normal boiling point of diethyl ether?
°C
The normal boiling point for diethyl ether is 36 °C. A low temperature!
8. Which substance has the strongest intermolecular attractive forces?
Water has the strongest intermolecular attractive forces.
Briefly explain your answer.
The stronger the intermolecular attractive forces that occur in a liquid the lower the vapor pressure the liquid will have at a given temperature. Looking at the figure above and looking at a tmperature all three liquids have a vapor pressure (less than 36 °C), it is evident that water has the strongest intermolecular attractive forces, since water has the lowest vapor pressure of the three liquids.
9a. Using the plot above, what is the vapor pressure of water at 20 °C?
mmHg
The vapor pressure of water at 20 °C is about 20 mmHg.
b) Using the plot above, what is the vapor pressure of water at 40 °C?
mmHg
The vapor pressure of water at 20 °C is about 50 mmHg.
10. When you heat a sample of water, why does the vapor pressure increase?
Heating water will increase the kinetic energy of the water molecules. Adding heat will mean that more molecules of water will have sufficient energy to escape the liquid phase into the vapor phase, and increasing the equilibrium vapor pressure.
10. Is there anything about the questions that you feel you do
not understand? List your concerns/questions.
11. If there is one question you would like to have answered in lecture,
what would that question be?

