Thank you for completing this BCE. You may want to print this page out and bring it to class so if you have any questions you can ask them.
BCE Response Page
I recommend you print out this page and bring it to class. Click
here to show a set of five student responses, randomly selected from
all of the student responses thus far, in a new window.
, here are your responses
to the BCE and the Expert's response.
Click on the Models360 link to open the page in a new tab. In the right side of the screen click on the Solids tab. In the new screen click on the Primary Metallic Solids button on the left side of the screen to reveal a list of structures. Click on the PC (Primitive cubic) structure to see a simple cubic structure and to rotate the structure. Once the page are satisfactory continue with Q1.
1. In the PC structure select small radii to see a similar image as shown below (although the color is different).
NOTE: You can move the image by placing the mouse in the Jmol viewing window then clicking and hold the button down and moving the mouse.) Move the image around a little so you can see the cubic nature of the bounding box and the eight atoms. You can switch between the small radius and large radius views. Measure the distance from the center of one of the atoms to the center of the adjacent atom on the same edge. To measure the distance between the two atom be sure the mouse is in the center of the first atom and double click the mouse (you may see the cursor change from an arrow to a cross), then move the mouse to the center of the adjacent atom and double click again. A distance in nanometer should appear along a dotted line between the two atoms. See the figure below.)
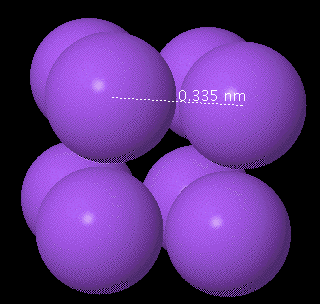
Enter the distance between the two centers.
nm
0.335 nm
2. Convert the distance from nanometers to meters. (1 nm = 1 x 10-9 meters)
meters
0.335 nm * (1 x 10-9 meters/1 nm) = 3.35 x 10-10 meters
3a. Knowing the distance between the two atoms corresponds to one edge of the cube. Calculate the volume of the cube. (NOTE: Calculate the volume in cubic meters, not cubic nanometers.)
m3
(3.35 x 10-10 meters)3 = 3.76 x 10-29 meters3
b) Is this a big number or a small number?
Small number
4. Since two atoms are just touching each other on this cube edge, and knowing the edge length of the cube, what is the radius of one of the atoms?
m
(3.35 x 10-10 meters/2 = 1.68 x 10-10 meters
5. Calculate the volume of the atom using the relationship for the volume of a sphere (V = 4/3*pi*r3)
m3
4/3 * 3.1415 * (1.68 x 10-10 meters)3= 1.97 x 10-29 meters3
6. Assuming that each corner atom (their are eight corner atoms) contributes one-eighth of its volume to the cube how many total atoms end up being inside the cube volume?
atoms
1 atom is in the cubic cell sincce eight corners each contribute 1/8th of an atom.
7. OK, this may be a tough question...using your answers in Q6a, Q8 and Q9 calculate the percent of empty space in the simple cube structure.
%
If we divide the volume occupied by the eight corner atoms that each contribute 1/8th to the cell by the total volume of the cell that will tell us the percentage of the cell that the atom occupies. Subtracting from 100 gives us the volume of empty space.
(1.97 x 10-29 meters3/3.76 x 10-29 meters3 ) * 100 = 52.4% occupied
so
100 - 52.4% = 47.6% empty space
8. Is there anything about the questions that you feel you do
not understand? List your concerns/questions.
9. If there is one question you would like to have answered in lecture,
what would that question be?


