Welcome to Acid/Base chemistryweb-site
"Acid/Base reaction is a simple fundamental reaction that will enable you to see how chemists use curved arrows to represent mechanisms of reactions and how they depict the processes of bond breaking and bond making that occur as molecules react."
Acids and Bases may be defined in several ways. the definition suggested in 1923 by the Danish chemist Johannes Bronsted(1879-1947) and the english chemist Thomas Lowry (1874-1936). in the Bronsted-Lowry definition. An Acid is any chemical that donates a hydrogen ion, H+, and a Base is any chemical that accepts a hydrogen ion, as A hydrogen ion consists of one electron surrounding a one-proton nucleus, a hydrogen ion formed from the loss of an electron is nothing more than a lone proton.
so an acid is a chemical that donates a proton and a baseis a chemical that accepts a proton.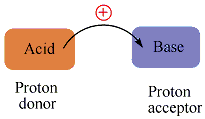
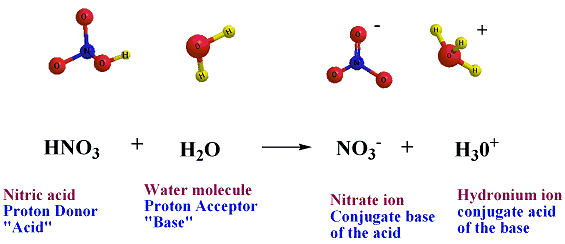
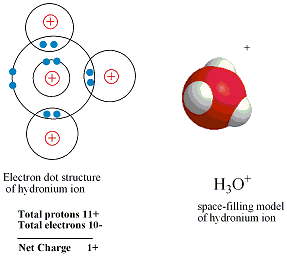
Hydrogen chloride donates Hydrogen ion to one of the nonbonding electron pairs on a water molecule , resulting in a third hydrogen bonded to the oxygen. in this case, Hydrogen chloride behaves as an acid (proton donor) and water behaves as a base (proton acceptor).the products of this reaction are a chloride ion and a hydronium ion , H3O+, which, is a water molecule with an extra proton.
when added to water, ammonia behaves as a base as its nonbonding electrons accept a hydrogen ion from water, which, in this case, behaves as an acid:
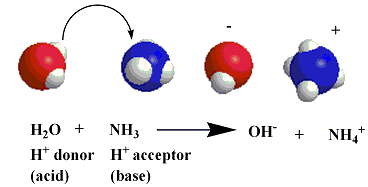
this reaction results in the formation of an ammonium ion and a hydroxide ion, whcih, is a water molecule without the nucleus of one of the hydrogen atoms.

an important aspect of the Bronsted-Lowry definition is that it recognizes
acid-base as a behavior. for example, hydrogen
chloride behaves as an acid when mixed with water,
which behaves as a base when mixed with water, which
under this circumstance behaves as an acid. Because acid-base
is seen as a behavior, there is really no contradiction when a chemical
likewater behaves as a base (accept H+) when
mixed with hydrogen chloride and as an acid
(donate H+) when
mixed with ammonia.
the products of an acid-base reaction can also behave as acids or bases. an ammonium ion can donate a hydrogen ion back to a hydroxide ion to re-form ammonia and water:
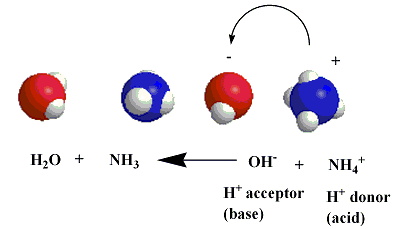
forward and reverse acid-base reactions proceed simultaneously and can therefore be represented as occuring at the same time by using oppositely facing arrows:
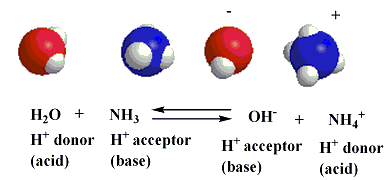
when the equation is viewed from left to right, the ammonia behaves as a base because it accepts a hydrogen ion from the water, which therefore acts as an acid. viewed in the reverse direction, the equation shows that the ammonium ion behaves as an acid because it donates a hydrogen ion to the hydroxide ion which therefore behaves as a base.
Navigation menus
- Overviews
- Tutorials
- Experiments
- Questions
- Home
Bronsted-Lowry Acid-Base Experiments
- Litmus paper experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Indicator experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.
- Acid-Base pH meter titration Experiment
Wide selection of Brother Ink Cartridges - Acid-Base Titration experiment
Web Hosting for 10 Websites