Welcome to The Acid/Base chemistryweb-site
Example Showing the Proton donation in the Bronsted-Lowry Acid Base Reactions
Consider the reaction between nitric acid which acts as an (acid) and water molecule which acts as a base (base)

Nitric acid, HNO3 is a polar acidic molecule, The bonding pair of electrons will be attractd to the oxygen atom-as oxygen atom is more electronegative than hydrogen atom-leaving the Hydrogen atom without electrons (Hydogen atom will become a proton), so Nitric acid will work as proton donor.
while Water molecule which is a polar molecule, will be a proton Acceptor
due to the strong dipole-dipole interactions between the Hydrogen of the nitric acid and the oxygen atom of water.
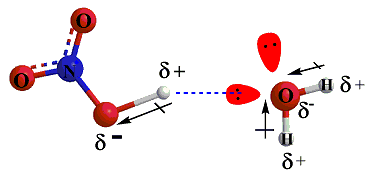
The Dipole-Dipole force is strong enough to pull the hydrogen atom away from the nitiric acid as H+- (H+) ion is a proton with no valence electron -towards the oxygen atom of the water molecule, leaving NO3- anion.

As H+ forms a covalent bond with the oxygen atom of the water molecule, using one of the water molecule lone pairs, to form H3O+.
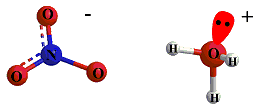
By the end of the reaction we have Hydronium ion,(Conjugate acid of the water molecule "Base"), and Nitrate Ion (Conjugate base of the nitric acid).
Acid And Base Topics
- Acid-Base identification Activity
- Acid-Base Identification activity
- Acid And Base Topics
- Acid/ Base characteristics/ Arrhenius theory.
- Acid/Base Arrhenius theory limitations.
- Bronsted-Lowry A/B theoryI.
- Bronsted-Lowry A/B theoryII.
- Lewis Acid/Base theory
- Acid And Base Topics
- Acid-Base strength I.
- Acid-Base strength II.
- Bronsted-Lowry QuestionII
- Bronsted-Lowry questionII
- Quiz
- Acid-Base Quiz
Pre-classroom activity and Experiments
- Litmus paper experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Indicator experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.
- Acid-Base pH meter titration Experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Acid-Base Titration experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.