Welcome to The Acid/Base chemistryweb-site
"Acid/Base reaction is a simple fundamental reaction that will enable you to see how chemists use curved arrows to represent mechanisms of reactions and how they depict the processes of bond breaking and bond making that occur as molecules react."
Acids and Bases have different ability to accept and donate protons,Acids and Bases have different strengths. Acid strength, is its ability to donate a proton.
the stronger an acid, the more easily an acid gives up a proton.the weaker is its conjugate base, the less easily for this conjugate base to accept a proton.the stronger the base, the more easily the base accepts the proton.the weaker is its conjugate acid, the less easily its conjugate acid gives up a proton.
The stronger the acid, the weaker is its conjugate base; the stronger the base, the weaker is its conjugate acid.
the stronger acid reacts with the stronger base to produce a weaker conjugate acid and conjugate base.
the reaction proceed essentially 100% to the right.
HCl(aq)(stronger acid) + H2O(l)(stronger base) -----> H3O+(aq)(weaker conjugate acid) + Cl-(aq)(weaker conjugate base)

from the previous reaction Hydrogen chloride (HCl) which is a strong acid,completely ionizes in water., therefore, strong acid completely ionizes in water, and it will give all its protons to water. its conjugate base has a negligible tendency to protonate in solution.while in the reaction between acetic acid-HC2H3O2- (weak acid) with water, acetic acid is partly ionized in aqueous solution, and so exist in the solution as a mixture of acid molcules and their ions.conjugate base of weak acid is weak base.
CH4 is considered to have negligible acidity. as it has hydrogen but doesn't demonstrate any acidic behavior in water, its Conjugate base is strong base. reacting completely , abstracting protons from water molecules to form OH-.
some common acids ranked in order of decreasing strength are shown in table (3-1). the stronger the acid, the higher it ranks in the table. the three common strong acids are listed at the top, but even among the weak acids there is variation in their strengths.
Go To Acid-Base Table of Common Bronsted-Lowry Acids and Bases
Any acid has a corresponding conjugate base which it can form by the loss of a proton. the conjugate base of each acid is included in table (3-1). the base are listed in the right-hand column. the conjugate bases are also listed according to relative strength, but they are listed with the weakest at the top and the strongest at the bottom. this arrangement makes sense because the stronger an acid, the weaker its conjugate base, and the weaker an acid, the stronger its conjugate base.
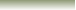
From table (3-1) water is listed as a very weak acid at the bottom of the list of acids and as a weak base near the top of the lsit of bases. any of the strong acids, when mixed with water, reacts to give hydronium ions, H3O+, and the conjugate base.
using HXfor a strong acid this reaction is represented as
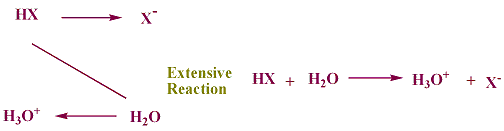
thus, the hydronium ion is the strongest acid that can exist in water.
Weak acids are molecular substance, H2S is a molecular weak acid, HS-(Hydrogen sulfide ion) is a weak acid. HA is a weak acid, which will slightly react with water.
Example, consider that a solution of Hydrogen sulfide contains H2S as the major species since it only reacts with water to a slight extent.

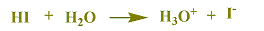
CO2 has no proton, but when dissolve in water gives an acidic solution of carbonated water, this is why we have CO2 is included in the table as CO2 + H2O (H2CO3)- carbonic acid-.
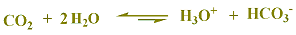
from table (3-1), most common bases are negatively charged ionic species. the only exceptions are water and ammonia, NH3. water and ammonia are molecular bases. in a water solution of any base, the base is the major speices. when a base, as represented as B, that is weaker than OH- is in water, it reacts only slightly with water.
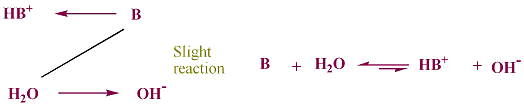
for example, in a solution of ammonia, NH3(aq) is the major species.
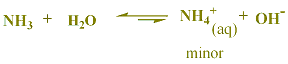
for a solution of an ionic compound, the major species are the ions. in a solution of NaOH, Na+ and OH- are the major species.
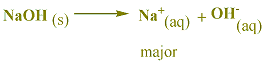
a solution of NaOH is viewed as a strong base since it contains relatively high concentrations of hydroxide ion. hydroxide ion is the strongest base that can exist in water.
factors affecting acid strength
A) H-X bond polarity:
* when the molecule contains a hydrogen atom, it will transfer a proton if the H-X bond is polarized, and X atom is more polar than the H atom.
* for ionic hydrides, the hydrogen atom carries a negative charge, and acts as a proton acceptor. example: KH H-(aq) + H2O -----> H2(g) + OH-(aq)
* nonpolar H-X bonds, produce neither acidic nor basic aqueous solution, example: H-C in CH4(methane)
B) H-X bond Strength:
* H-X bond strength is important in determining the strength of an acid, the more polar the H-X bond is, the weaker is the acid. example, H-F bond is the most polar H-X bond, energy required to dissociate HF into H and F atoms is higher than it is for the other hydrogen halides, so HF is a weak acid.
C) Stability of the conjugate base, X- :
* the higher the stability of conjugate base, X-, the stronger is the acid
in binary acids (which are compounds that contains hydrogen and just one other element).
* the H-X bond strength increases as X element increases in size down a group , the bond strength decreases , and the acidity increase. (HCl is stronger than HF, and H2SO4 is stronger than H2O.
base strengths increases when going up in the same group.
for Binary acids, the acidity increases, acidity increases as the electronegativity of the element X increases. so acidity increases when moving from left to right in the same row, and base strength increase when moving from right to left .
HF is a weak acid, H2O is negligible, NH3 is weak base, CH4 has no acidic neither basic properties. in CH4 C-H bond is nonpolar, so CH4 doesn't show any acidic or basic properties. and so can't form H+ + CH3- ions., while in NH3 , N-H bond is polar , but the presence of a nonbonding pair of electrons on the N atom, helps NH3 to act as a proton acceptor, (a base).
Oxyacids
are acids in which OH groups, and additional oxygen atoms are bound to a central atom.
A3-X-O-H if X is a metal, like Na, Mg, k.
the pair of electrons shared between X and O is transferred to oxygen because of the low electronegativity of the metal atoms, anionic compound containing OH- is formed, such compounds are sources of OH- ions and behave as bases.
if X is a nonmetal, the bond to O is covalent. as the electronegativity of X increase, the acidity of the substance will increase.
* as electron density is drawn toward X, and OH bond becomes weaker and tends to lose H+.
* the conjugate base is an anion, which stability increases by increasing the electronegativity of X.
Br- O- H  BrO- + H+
BrO- + H+
from this equation, as Br atom has an electronegativity of 2.8 , so the electron density is shifted towards Br atom.
the order of decreasing acidity: HClO > HBrO > HIO as electronegativity of Cl is 3, while that of Br is 2.8, and that of I is 2.5.
by increasing the number of oxygen atoms attached to X atom, which also leads to an increase in the oxidation number of the central atom, the strength of the acid will increase, by adding more electronegative oxygen atoms, which will pull electron density from the O-H bond. by increasing its polarity.
carboxylic acids: with the formula, CH3-C(O)-OH
Acidity of carboxylic acid, due to the presence of the second oxygen atom, which attracts the electron density from OH bond, increasing its polarity and help to increase solubility of conjugate base.
the conjugate base is stabilized by resonance. acid strength of carboxylic acid increases, by increasing number of electronegative atoms. CF3COOH > CH3COOH
Acid And Base Topics
- Acid And Base Topics
- A/B Characteristics And Arrhenius Theory.
- Acid/Base Arrhenius theory limitations.
- Bronsted-Lowry A/B theoryI.
- Bronsted-Lowry A/B theoryII.
- Lewis Acid/Base theory
- 22-08-06Fitted tabs NO images
- 28-07-06Simple FOUR level flyout
- 27-07-06Simple FOUR level dropdown
- 28-06-06Vertical slide
- 11-06-06Fly-out examples
- LAYOUTS
- 07-01-06100% 'background' image
- MOZILLA
- 03-01-06Shadow text
- OPACITY
- 08-01-06Partial opacity II
Bronsted-Lowry Acid-Base Experiments
- Litmus paper experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Indicator experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.
- Acid-Base pH meter titration Experiment
Wide selection of Brother Ink Cartridges - Acid-Base Titration experiment
Web Hosting for 10 Websites