Welcome to The Acid/Base chemistryweb-site
In this website we will discuss the chemistry of Acids and Bases, as we know Acids and Bases can be considered as the most important chemicals in our lives, so we will take a closer look at Acids and Bases and their general characteristics. we will start with the general characteristics of Acids.
General characteristics of Acids.
General characteristics of Bases
Postulated Theories for Acid-Base Definition
There are three theories talked about Acid-Base definitions. These theories started with Svante Arrhenius, then followed by Bronsted-Lowry Acid-Base theory, and the last theory was for Lewis Theory
the chemical work started earlier with the swedish chemist, in 1884, Svante Arrhenius, proposed the first scientific definition of acid and base.
Arrhenius defined an acid as any substance that contains hydrogen and when dissolved in aqueous solution produces hydrogen ions (Protons)(H+) and anions.so it will increase the Hydrogen ion concentration in water by the proton donation.
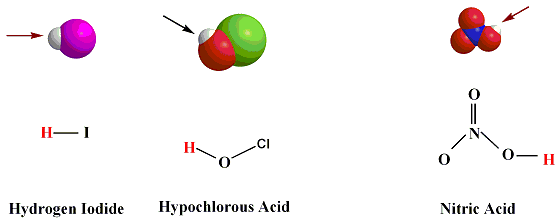
In Writing the acid formula, we need to write the formula of the H atom first before the anion formula.
examples of acids. HF, HI, HCl, H3O+, H3PO4, HNO3, are all acids.
while, NO3-, PO43- are not acids.
The effects in color change from blue litmus paper to red ,the sour taste of the Acidic compounds,the reaction with active metals and evolution of hydrogen gas due to the presence of hydrogen cation (H+) in the aqueous medium.

Arrhenius definition of a base or (Metal Hydroxide) as a substance that contains a metal and Hydroxide (OH-) group and Produces hydroxide ions (OH-) in the aqueous solution. so it will increase the OH- concentration in water. can be represented by the formula M(OH)n. where M stands for a metal atom, and (OH-) is the Hydroxide Ion.
NaOH  Na+ + OH-
Na+ + OH-
Acid And Base Topics
- Acid And Base Topics
- Acid/ Base Strengths.
- Acid/Base Arrhenius theory limitations.
- Bronsted-Lowry A/B theoryI.
- Bronsted-Lowry A/B theoryII.
- Lewis Acid/Base theory
- A/B Characteristics And Arrhenius Theory.
- Acid/Base Arrhenius theory limitations.
- Bronsted-Lowry A/B theoryI.
- Bronsted-Lowry A/B theoryII.
- Lewis Acid/Base theory
- LAYOUTS
- 07-01-06100% 'background' image
- MOZILLA
- 03-01-06Shadow text
- OPACITY
- 08-01-06Partial opacity II
Bronsted-Lowry Acid-Base Experiments
- Litmus paper experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Indicator experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.
- Acid-Base pH meter titration Experiment
Wide selection of Brother Ink Cartridges - Acid-Base Titration experiment
Web Hosting for 10 Websites