Sample Problem:
Calculate the temperature the reaction,
2H2(g) + O2(g) ----> 2H2O(g)
becomes nonspontaneous.
Answer:
We need the value of  H
and
H
and  S to solve this problem. We've
already calculated
S to solve this problem. We've
already calculated  H and
H and  S
for the reaction in the previous problem.
S
for the reaction in the previous problem.
So to answer this question we will consider the Gibbs-Helmholtz
equation;
 Gsystem
=
Gsystem
=  Hsystem - T
Hsystem - T Ssystem
Ssystem
which relates  H and
H and
 S for the reaction to
S for the reaction to  G.
In the previous problem we determined the following values for
G.
In the previous problem we determined the following values for  H,
H,
 S and
S and  G;
G;
 H = - 484 kJ
H = - 484 kJ
 S =
S = 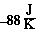
 G = - 458 kJ
G = - 458 kJ
So at 25 degree Celsius (298 K) the reaction is definitely spontaneous.
If we change the temperature say to 400 K (a higher temperature)
we can calculate  G at the higher
temperature using the Gibbs-Helmholtz equation,
G at the higher
temperature using the Gibbs-Helmholtz equation,
 Gsystem
=
Gsystem
=  Hsystem - T
Hsystem - T Ssystem
Ssystem
 Gsystem
= -484 kJ - 400 K(
Gsystem
= -484 kJ - 400 K(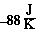 )
)
 Gsystem
= -484 kJ + 35.2 kJ = -347 kJ
Gsystem
= -484 kJ + 35.2 kJ = -347 kJ
You can see increasing the temperature causes the  G
to become more positive (less negative). We could continue this way, entering
a higher temperature and calculating
G
to become more positive (less negative). We could continue this way, entering
a higher temperature and calculating  G.
But the easiest way is to realize that for the reaction to become nonspontaneous
G.
But the easiest way is to realize that for the reaction to become nonspontaneous
 G must go to 0. So we just need to
set
G must go to 0. So we just need to
set  G = 0 and calculate the T that
would be the temperature at which the reaction becomes nonspontaneous.
G = 0 and calculate the T that
would be the temperature at which the reaction becomes nonspontaneous.
So assuming  G = 0
G = 0
 Gsystem
=
Gsystem
=  Hsystem - T
Hsystem - T Ssystem
Ssystem
0 = -484 kJ - T(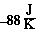 )
)
484 kJ = T(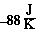 )
)
T = 5500 K (pretty high temperature)
Now while the math is pretty straightforward we have to realize
this is only an approximation. One of the problems we have is this calculation
assumes  H and
H and  S
do not change with change in temperature. That assumption is not too bad, and
works for small temperature changes, say, 200 - 300 K, but is not very good
for large temperature changes as in this case. So while we calculate this temperature,
we must realize that it is only an approximation. The discussion that would
be required to cover what must be done to more accuratley calculate this temperature
is beyond the scope of this course. When you pay more money, you can take P-Chem
and they will teach you how to do this calcualtion more accurately.
S
do not change with change in temperature. That assumption is not too bad, and
works for small temperature changes, say, 200 - 300 K, but is not very good
for large temperature changes as in this case. So while we calculate this temperature,
we must realize that it is only an approximation. The discussion that would
be required to cover what must be done to more accuratley calculate this temperature
is beyond the scope of this course. When you pay more money, you can take P-Chem
and they will teach you how to do this calcualtion more accurately.