Welcome to The Acid/Base chemistryweb-site
Example showing the proton donation in the Bronsted-Lowry
acid base reactions
Consider the reaction between nitric acid which acts
as an acid and water molecule which acts as a base.

The reaction between Nitric acid and water takes four steps:
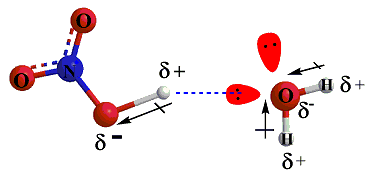

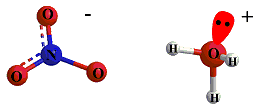
Acid And Base Topics
- Acid-Base identification Activity
- Acid-Base Identification activity
- Acid And Base Topics
- Acid/ Base characteristics/ Arrhenius theory.
- Acid/Base Arrhenius theory limitations.
- Bronsted-Lowry A/B theoryI.
- Bronsted-Lowry A/B theoryII.
- Lewis Acid/Base theory
- Acid And Base Topics
- Acid-Base strength I.
- Acid-Base strength II.
- Bronsted-Lowry QuestionII
- Bronsted-Lowry questionII
- Quiz
- Acid-Base Quiz
Pre-classroom activity and Experiments
- Litmus paper experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Indicator experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.
- Acid-Base pH meter titration Experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Acid-Base Titration experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.


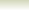
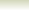
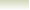
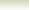




 NO3-
+ OH-
NO3-
+ OH-