Formation reactions
Combustion reactions
Neutralization reactions
Phase changes
A formation reaction is a reaction that produces one mole of a compound from its elements in their standard state (their state at 25 degrees Celsius and 1 atmosphere). We can look at a list of
Hof reactions in your textbook. Doing a quick scan you will see the
Hof of most compounds is negative, that is the formation reaction is exothermic. This is probably not too surprising since few elements are found naturally occurring. The noble gases (He, Ne, Ar, Kr, Xe and Rn); the noble metals (Cu, Ag, Au) are found in their elemental state. Another element that is found in its elemental state is nitrogen. We also know oxygen exists in its elemental state, but that is due to the balance of it formation (plants), and that most elements that would react with oxygen, have reacted with oxygen. So most formation reactions are exothermic. There is one group that most of the
Hof the compounds is positive, endothermic. This group contains nitrogen. Since a formation reaction forms a compound from its elements in their standard state, to form a compound containing nitrogen we must use N2 as a reactant. N2 has a triple bond and it takes more energy to break the triple bond than is recovered in the bonds to nitrogen in the product compound. Looking more carefully at the nitrogen containing compounds, most of the
Hof of oxygen containing compounds of nitrogen are endothermic.
Most combustion reactions, a compound reacts with oxygen from the air, are exothermic. When methane is combusted the heat produced is used to cook. Natural gas is principally methane. Butane is used in lighters and its combustion is exothermic.
Most neutralization reactions, a reaction between an acid and a base, are exothermic. However, there is one very important exception to this rule (discussed below).
You should have some intuition about phase changes, the conversion of a pure substance from one phase to another. The conversion from solid to liquid and liquid to gas are both endothermic. We must add heat to convert water from the liquid to the vapor phase. The phase change from gas to liquid and from liquid to solid are both exothermic. Anyone burned by steam understands how exothermic the phase change from gas to liquid is.
This reaction is between barium hydroxide and ammonium chloride. Both of these reactants are white crystalline solids. The two white solids are mixed together in a erlenmeyer flask. Before the reaction begins the two solids are at room temperature as is the flask. When the two solids are mixed the first observation is the two solids form a slurry, as a liquid is formed. This is very interesting, but not surprising when you are told water is a product of the reaction. The second observation occurs when the flask is picked up. The temperature has fallen when the two reactants are mixed to a very low temperature. The reaction is;Ba(OH)2.8H2O(s) + 2NH4SCN(s) ---> Ba(SCN)2(aq) + 2NH3(g) + 10H2O(l)As the two white solids are mixed we observed how the temperature of the reaction changed. The temperature is falling-it is getting cooler. Notice also the change which has occurred in the flask. The two solids have changed and we now have a liquid. If we could smell the reaction vessel it would smell of ammonia. Heat is a reactant, it is absorbed by the reactants.(Click on the picture on the right to view the reaction.) |
|---|
the second law of thermodynamics states that thermodynamically favored natural processes are accompanied by an increase in the entropy of the universe.
the third law of thermodynamics says that the entropy of any pure substance at absolute zero is zero.
Substance |
Entropy (joules Kelvins-1) |
C(s) (graphite) |
5.69 |
C(s) (diamond) |
2.44 |
Al(s) |
28.3 |
Ca(s) |
41.6 |
Ba(s) |
62.5 |
Br2(l) |
152 |
H2(g) |
130.6 |
N2(g) |
191 |
O2(g) |
205 |
NO(g) |
210.6 |
F2(g) |
202.7 |
H2O(l) |
69.9 |
H2O(g) |
189 |
CO2(g) |
213.6 |
NH3(g) |
193 |
CH4(g) |
186 |
SO2(g) |
248.5 |
SO3(g) |
256.2 |
C2H6(g) |
229.5 |
C3H8(g) |
269.9 |
HF(g) |
174 |
HCl(g) |
187 |
HBr(g) |
199 |
HI(g) |
206 |
NaCl(s) |
72.3 |
NaCl(aq) |
115.5 |
NaNO3(s) |
116.5 |
NaNO3(aq) |
207 |
NaOH(s) |
64.5 |
NaOH(aq) |
49.8 |
physical states - looking at the table above in general the entropy values for gases are larger than entropy values for liquids and for solids. Particles in the gas phase are more dispersed in energy and in position compared to a particles in the liquid phases and both liquid phase and gas phase are more dispersed than a solid. So we would expect that chemical reactions producing more gases compared to the reactants will have a positive
S.
temperature - As discussed earlier about the third law of thermodynamics, the higher the temperature the greater the energy for the substance/system, and the more dispersed that energy can become.
solution process - dissolving solids in water/solvent generally leads to greater dispersal. So comparing NaCl(s) to NaCl(aq) in the aqueous solution the ions are more dispersed compared to the ions in the solid. However, there are some exceptions to this rule, some ions have a very large charge and when added to water, order the water molecules resulting in a more ordered state, or reducing the extent that water molecules are dispersed in the solution. Al3+ is an example of an ion that when dissolved in water produces a more ordered system. When gases dissolve in water the
S is always negative. For example, O2(g) has a higher entropy than O2(aq).
molecular complexity - this is a more complicated issue. From the table above we can see some trends, for example, HF, HCl, HBr and HI. The entropy value increases going down the group. The more electrons in the molecule of similar structure the greater the entropy. We can also see a similar trend for CH4(g), C2H6(g), and C3H8(g). In this case the molecule is getting more complex as CH2 groups are added.
In a reaction if the products are more dispersed (in energy and position) compared to the reactants the sign of
S is positive (+).
If the products are less dispersed (in energy and position) compared to the reactants the sign of
S is negative (-).
1. Phase changes-this is the most important factor when determining the
S for a reaction. If the product phase(s) are phase(s) where particles are more dispersed compared to the reactants
S will be positive.
Example: What is the sign of
S for the reaction;
2HgO(s) ----> 2Hg(l) + O2(g)
S is positive (+) since a solid (reactants) is forming a liquid and a gas (products).
2.
n (change in moles)-this is the next most important factor when determining
S for a reaction. So if the chemical change can not be determined in terms of phases, doispersal is increased when there are more possibilities of where an element can be. So in a reaction the more moles of substance there are the greater the entropy (disorder).
Example: What is the sign of
S for the reaction;
In this reaction the products and the reactants have the same phase, so we can not use phase change to determine the
S. There are two moles of products and three moles of reactants. Since there are fewer moles of products the reaction
S is negative.
3. Molecular complexity-the last factor to consider, assuming there is no change in phase, and number of moles of product and reactants are equal, is the molecular complexity. The more atoms, the more variety of elements, and/or the more number of electrons all favor greater disorder.
Example: What is the sign of
S for the reaction;
H2(g) + Cl2(g) ----> 2HCl(g)
In this reaction the the product and reactants have the same phase and there are equal moles of reactants and products. Molecular complexity must be the criteria used to determine the
S. In this case HCl has great molecular complexity compared to H2 or Cl2 because the HCl has different elements. The difference in entropy between the reactants and products is small, but
S is still very slightly positive.
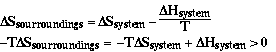
Sample Problem
Is the reaction below thermodynamically favored at 298 K?
a) 2H2(g) + O2(g) ----> 2H2O(g) Answer
Sample Problem
Calculate
G for the reaction,
Answer
Sample Problem:
Calculate the temperature the reaction,
2H2(g) + O2(g) ----> 2H2O(g)
becomes not thermodynamically favored. Answer