Welcome to The Acid/Base chemistryweb-site
The second scientists to talk about acid/base
theories were Dane Johannes
Bronsted (1879-1947) and Englishman Thomas M. Lowry (1874-1936)
independently. Bronsted and Lowry defined an acid
as any substance
that can give a hydrogen cation (proton donor) to another
substance
called a base. Bronsted-Lowry defined a base as any
substance that can
accept this hydrogen cation donated from
the acid (proton acceptor).
Bronsted-Lowry Base doesn't need
to contain OH- group.
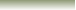
In the reaction Above we found that HNO3
and NO3-
are
Acid-Conjugate Base Pair and NH3 and NH4+
Acid-Conjugate
Base Pair.
Bronsted-Lowry Acid-Base Reaction (Proton-Transfer
Reaction) is
the reaction
in which a proton is transferred from
the acid (the proton donor)
to the base (the proton acceptor).
The Conjugate Acid-Base Pair: are two
molecules that differ from
each
others by only one proton. For an acid to work as an acid
it has to find the base that
will accept the proton. For a base to
work as a base it has to find an
acid that will donate the proton
to it. The Conjugate Acid. is the species formed when a proton
is added
to a base (base formula + proton ).
The Conjugate Base. is the species formed when a proton
is removed
from an acid ( acid formula - proton) . In the
Bronsted-Lowry Acid-Base reaction, a proton is transferred
from an acid to a base.
Bronsted-Lowry Acid-Base Reaction can represented as:
HA + B HB + A-
HB + A-
Where HA is the Bronsted-Lowry
acid, B is the
Bronsted-Lowry base, HB
is the conjugate acid,
A- is the
conjugate base.

Where does the OH- come from
in the ionization of bases as ammonia (NH3) ?
Ammonia is an Arrhenius base because adding it to water
increases the
[OH-] concentration. NH3 + H2O
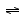 NH4+ + OH-
NH4+ + OH-
Ammonia is a Bronsted-Lowry base because it accepts a proton
from water.
Water acts as a Bronsted-Lowry acid, as it donates
a proton to the NH3
molecule.
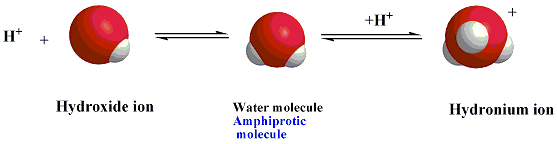
An amphoteric substance is any substance
that can act as an acid
(by donating a proton) when reacting with a base,
and as
a base (by accepting a proton) when reacting with an acid.
Example,
Water molecules.
Acid And Base Topics
- Acid-Base identification Activity
- Acid-Base Identification activity
- Acid And Base Topics
- Acid/ Base characteristics/ Arrhenius theory.
- Acid/Base Arrhenius theory limitations.
- Bronsted-Lowry A/B theoryI.
- Bronsted-Lowry A/B theoryII.
- Lewis Acid/Base theory
- Acid And Base Topics
- Acid-Base strength I.
- Acid-Base strength II.
- Bronsted-Lowry QuestionI
- Bronsted-Lowry QuestionI
- Quiz
- Acid-Base Quiz
Pre-Classroom Activity and Experiments
- Litmus paper experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Indicator experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.
- Acid-Base pH meter titration Experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Acid-Base Titration experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.