Welcome to The Acid/Base chemistryweb-site
The third scientist is Gilbert Newton Lewis,1923 ,and
his theory about
acid/base. The lewis theory deals with the way in which
a substance
accepts or donates a lone pair of electrons in an acid-base type
of reaction.
A Lewis Acid is an electron pair acceptor.A Lewis Base is an electron
pair donor.
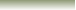
In The Reaction Below Between H+ and Ammonia.
Ammonia, NH3 acts as a Lewis Base (electron
pair donor) while
reacting with a metal cation or electron deficient atom like
(H+) which acts as Lewis Acid (electron pair
acceptor)to form
a complex ion
NH4+ .
The Lewis and Bronsted-Lowry theory are identical, because for a base
to accept a proton, a base must have an unshared pair of
electrons.
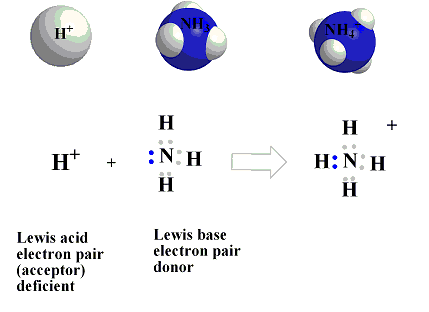

In The Reaction below between BF3 and NH3, BF3 acts
as a Lewis Acid with an incomplete octet of electrons,
so it has a vacant orbital in its valence shell in which
it will accept the lone pair of electrons from NH3,
a Lewis Base.
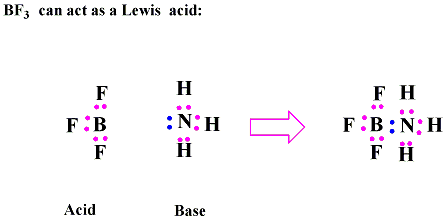
In the reaction below between Ferric Ion and
Cyanide ion.
Ferric acid acts as a Lewis Acid
or electron pair acceptor,
which will accept the electron pairs donated by the cyanide
ions which acts asa Lewis Base.
Species acting as Lewis acids (e.g. Cu2+ , Zn2+) need
not be Bronsted acids(proton donors). Species that act
as Lewis bases or (electron pair donor)
(e.g. H2O , NH3 , OH- ) are also Bronsted-Lowry
bases
or (Proton Acceptor) as it must have unshared pair
of electrons for binding the proton.
The Advantage of The Lewis Theory
Using Lewis theory can explain a wide variety of reactions,
including reactions with no proton transfer.
Acid And Base Topics
- Acid-Base identification Activity
- Acid-Base Identification activity
- Acid And Base Topics
- Acid/ Base characteristics/ Arrhenius theory.
- Acid/Base Arrhenius theory limitations.
- Bronsted-Lowry A/B theoryI.
- Bronsted-Lowry A/B theoryII.
- Lewis Acid/Base theory
- Acid And Base Topics
- Acid-Base strength I.
- Acid-Base strength II.
- Lewis Theory Question
- Lewis Theory Question.
- Quiz
- Acid-Base Quiz
Pre-Classroom Activity and Experiments
- Litmus paper experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Indicator experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.
- Acid-Base pH meter titration Experiment
This experiment enables you to titrate acid versus bases with the measurment of the pH. - Acid-Base Titration experiment
This experiment enables the titration of Acids versus Bases.