Welcome to The Acid/Base chemistryweb-site
Acids and bases differ in their ability to accept or donate protons,
which is affected by the degree of the acid and base strengths.
The most common strong acids are HCl, HBr, HI, HNO3, HClO3,
HClO4, H2SO4. Acid Strength, is controlled by its ability to donate
protons.
Strong acids are strong electrolytes which ionize completely
in solution.
The most common strong bases are ionic hydroxides of group 1A
and group 2A (NaOH, KOH, Ca(OH)2). Strong bases are strong
electrolytes which dissociate completely in solution.
e.g. NaOH (aq)
The oxide, hydride, nitride ions are stronger bases than hydroxides,
as they are able to withdraw a proton from water and generate OH-.
e.g. O2-(aq) + H2O (l)![]() 2 OH-(aq)
2 OH-(aq)
H+(aq) + H2O (l) ![]() H2(g) + OH-(aq)
H2(g) + OH-(aq)
The relationship between the relative strengths of
the acid and
its conjugate base, or between the base and its conjugate acid
can be summarized as follow:
The stronger an acid, the more easily it can give
up a proton.
The weaker
its conjugate base, the (harder it is) for this
conjugate base to
accept
a proton.The stronger the base,
the more easily the
base can accept
the proton.
The weaker its conjugate acid, the (harder)
its
conjugate acid gives up a proton.
The stronger the acid, the weaker is its conjugate
base; the stronger the base, the weaker is
its conjugate acid
See example below.
HCl(aq)(stronger acid) + H2O(l)(stronger
base) 
H3O+(aq)(weaker C/A) + Cl-(aq)(weaker
C/B )

From the previous reaction, hydrogen chloride (HCl)
which is a strong acid,
completely ionizes in water.
Therefore, strong acids completely
ionize in water, and give all their protons to water. their conjugate
base have a negligible tendency to protonate in a solution.
Weak acids are always in equilibrium, the solution
always exists
as a mixture of ions and un-ionized acid. In the reaction
between acetic
acid-HC2H3O2- (weak acid) with water,
acetic acid
is partly ionized in aqueous solution. The reaction exists in
the
solution as a mixture of acid molcules and their ions.
Conjugate
base of weak acid is weak base.
HA(aq) + H2O (l)
(partially ionized in aqueous solution )
Ka (acid-dissociation constant) = [H+][A-]/ [HA]
H2O is a pure liquid, not included in the Ka expression.
The larger the Ka, the stronger the acid, Ka is larger
since there are more ions present at equilibrium relative
to un-ionized molecules.
If Ka> 1, the acid is a strong acid,
which ionizes completely.
an equilibrium between the base and the resulting ions.
e.g. Weak Base + H2O(l) ![]() Conjugate Acid + OH-(aq)
Conjugate Acid + OH-(aq)
NH3(aq) + H2O (l) ![]() NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
The Base-dissociation constant (Kb) = [NH4+][OH-]/[NH3]
CH4 is considered to have negligible acidity. As it has hydrogen
but doesn't demonstrate any acidic behavior in water.
Acid-Base Strength Table: the stronger the acid, the
higher it
ranks in the table. The three common strong acids are listed
at the top, but even among the weak acids there is variation
in their
strengths.
Go To Acid-Base Table of Bronsted-Lowry Acids and Bases
All acids have a corresponding conjugate base which it can form
by the
loss of a proton. The conjugate bases of each acid is
included in the
acid-base table. Tthe bases are listed in the
right-hand column. The conjugate
bases are also listed according
to relative strength, but they are listed
with the weakest
at the top and the strongest at the bottom.
From the acid-base table. water is listed as a very
weak acid
(at the bottom of the list of acids) and as a weak base
near
(the top of the list of bases). any of the strong acids,
when mixed with water, reacts to give hydronium ions, H3O+,
and the conjugate base.
using HX for a strong acid this reaction is represented as

Thus, the hydronium ion is the strongest acid that can exist in water.
Weak acids are molecular substance: H2S is a molecular weak
acid,
and HS-(Hydrogen sulfide ion) is a weak acid. HA is a weak
acid,
which will slightly react with water.
For Example, consider that a solution of Hydrogen sulfide contains H2S
as the major species, since it only reacts with water to a slight extent.

CO2 is included in the table as
CO2 + H2O (H2CO3-)
carbonic
acid because CO2 has no protons, but when
it dissolves in water,
it results in an acidic solution of
carbonated water.
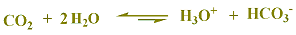
From the acid-base table, most common bases are
negatively charged ionic species. The only exceptions
are water and ammonia, NH3. As they are
molecular bases.
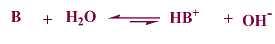
In a solution of ammonia, NH3(aq) is the major species.
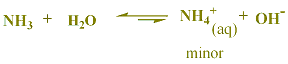
For a solution of an ionic compound, the major species
are the ions.
in a solution of NaOH, Na+ and OH-
are the major species.
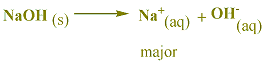
A solution of NaOH is viewed as a strong base since
it contains
relatively high concentrations of hydroxide
ion. hydroxide ion is the strongest base that can
exist in water.
Acid And Base Topics
- Acid-Base identification Activity
- Acid-Base Identification activity
- Acid And Base Topics
- Acid/ Base characteristics/ Arrhenius theory.
- Acid/Base Arrhenius theory limitations.
- Bronsted-Lowry A/B theoryI.
- Bronsted-Lowry A/B theoryII.
- Lewis Acid/Base theory
- Acid And Base Topics
- Acid-Base strength I.
- Acid-Base strength II.
- A/B strength Question
- Acid/Base strength question
- Quiz
- Acid-Base Quiz
Pre-Classroom Activity and Experiments
- Litmus paper experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Indicator experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.
- Acid-Base pH meter titration Experiment
this experiment explores the effect of different chemicals on the color change of Litmus paper, it also shows the difference between Bronsted-lowry acid and base. - Acid-Base Titration experiment
This experiment explores the effect of different chemicals on the color change of a n Acid-Base indicator.